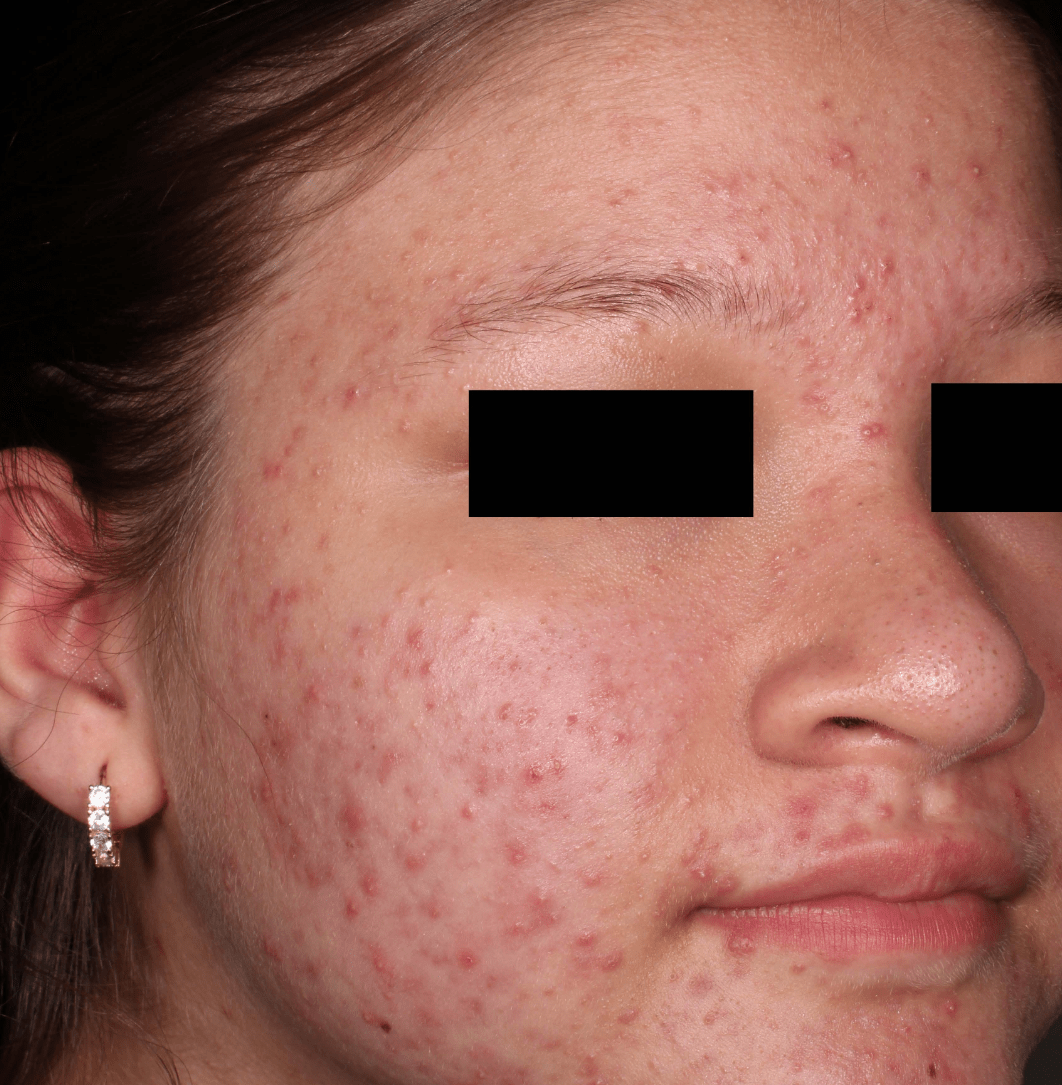
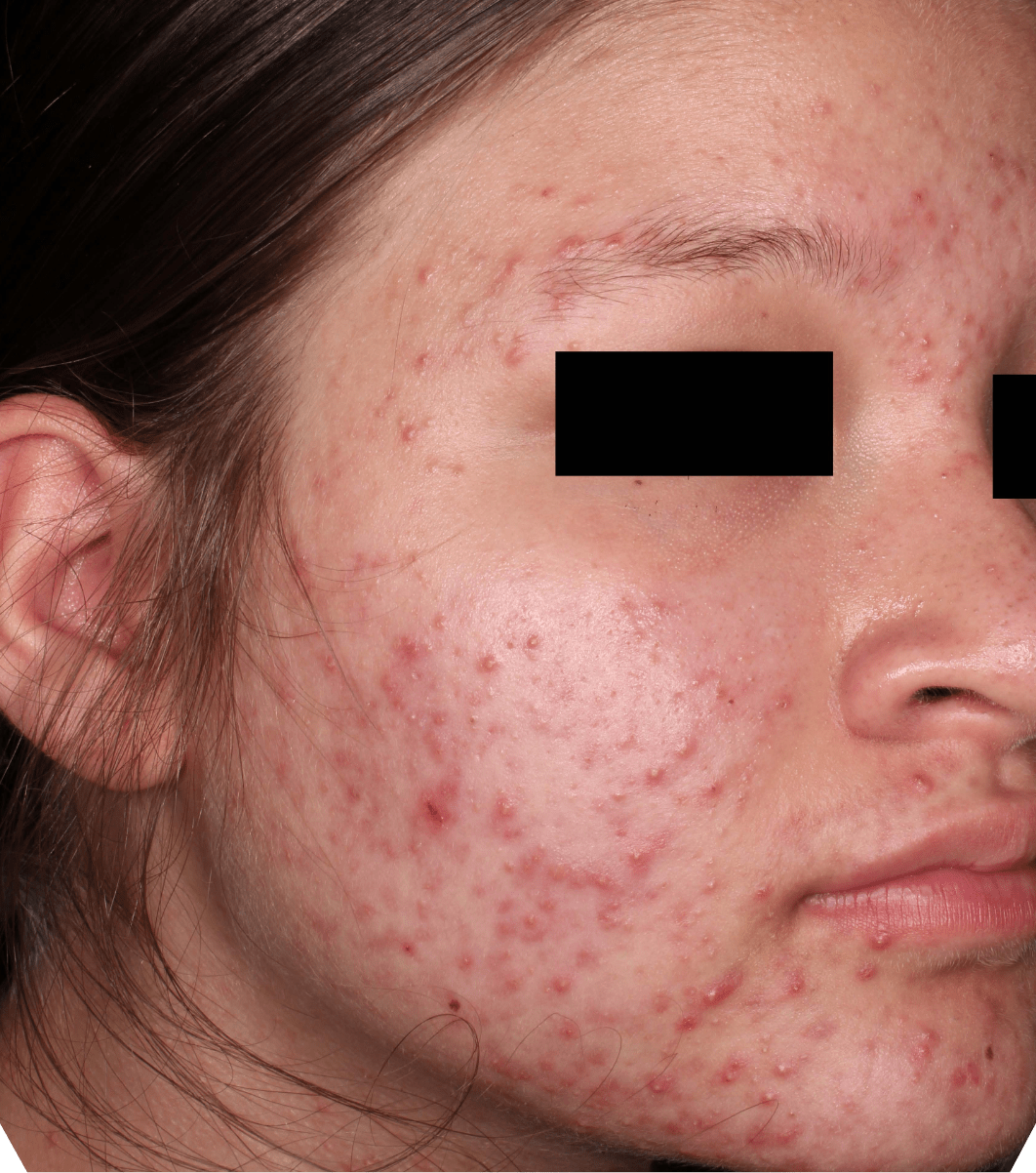
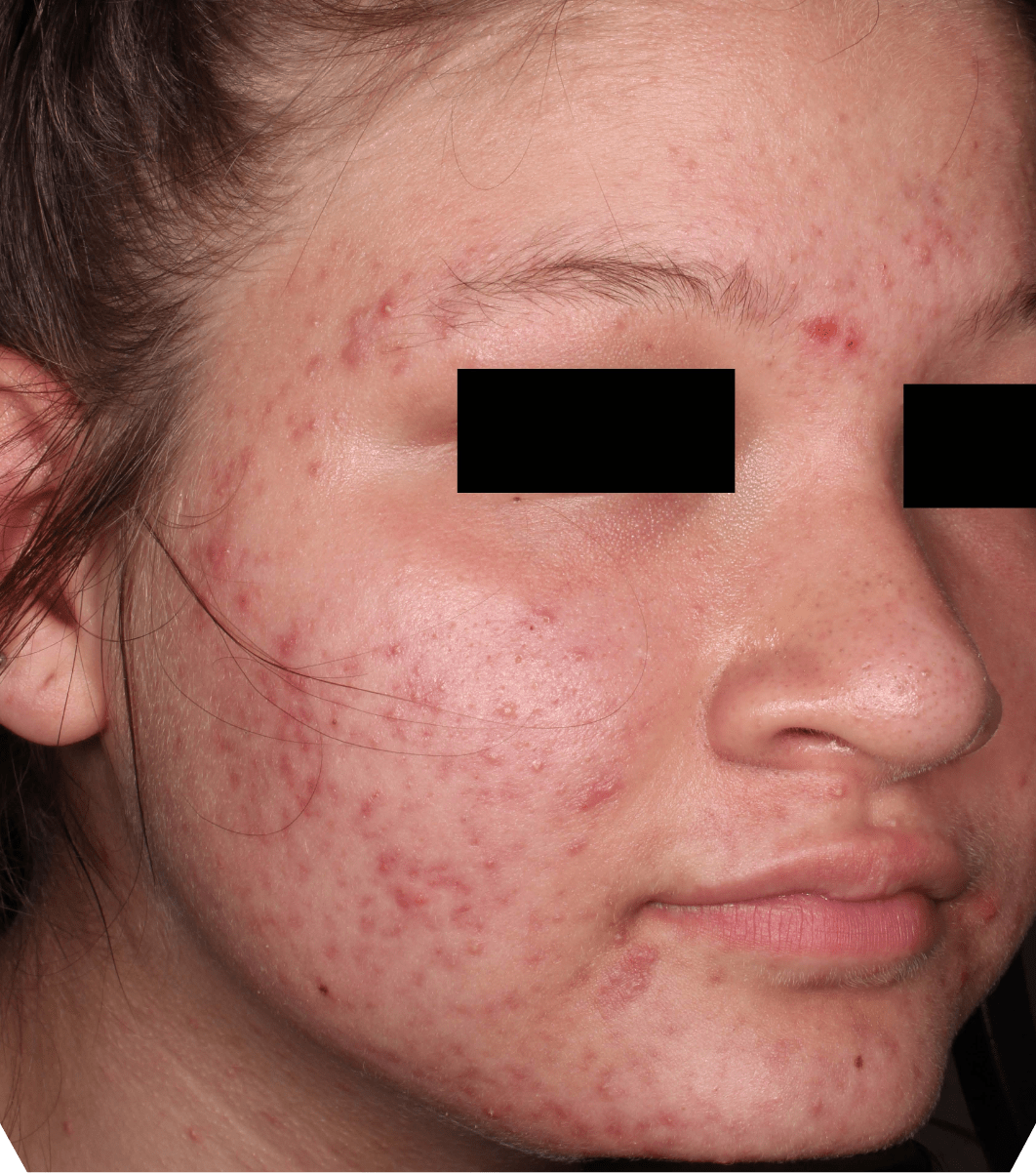
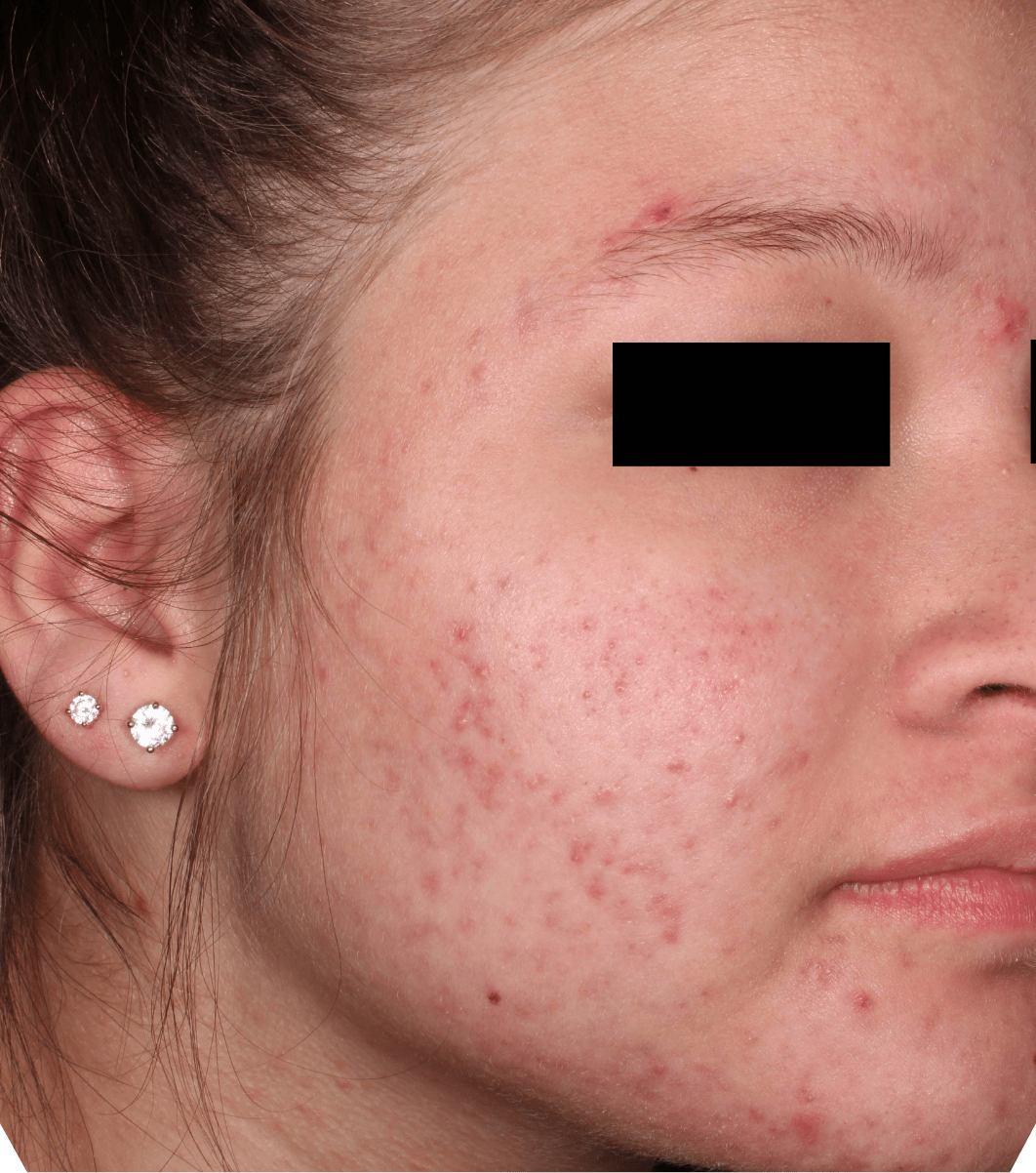
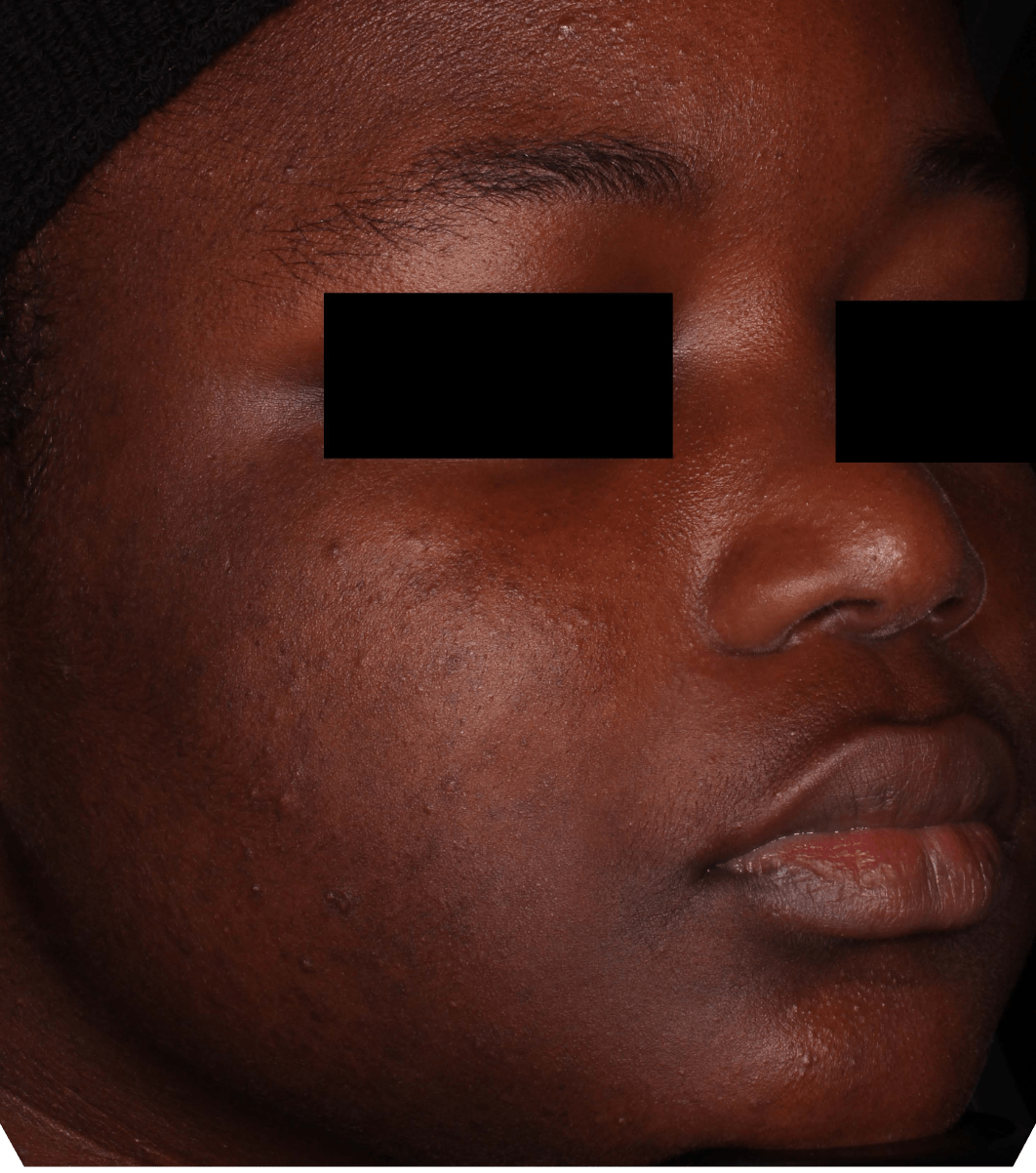
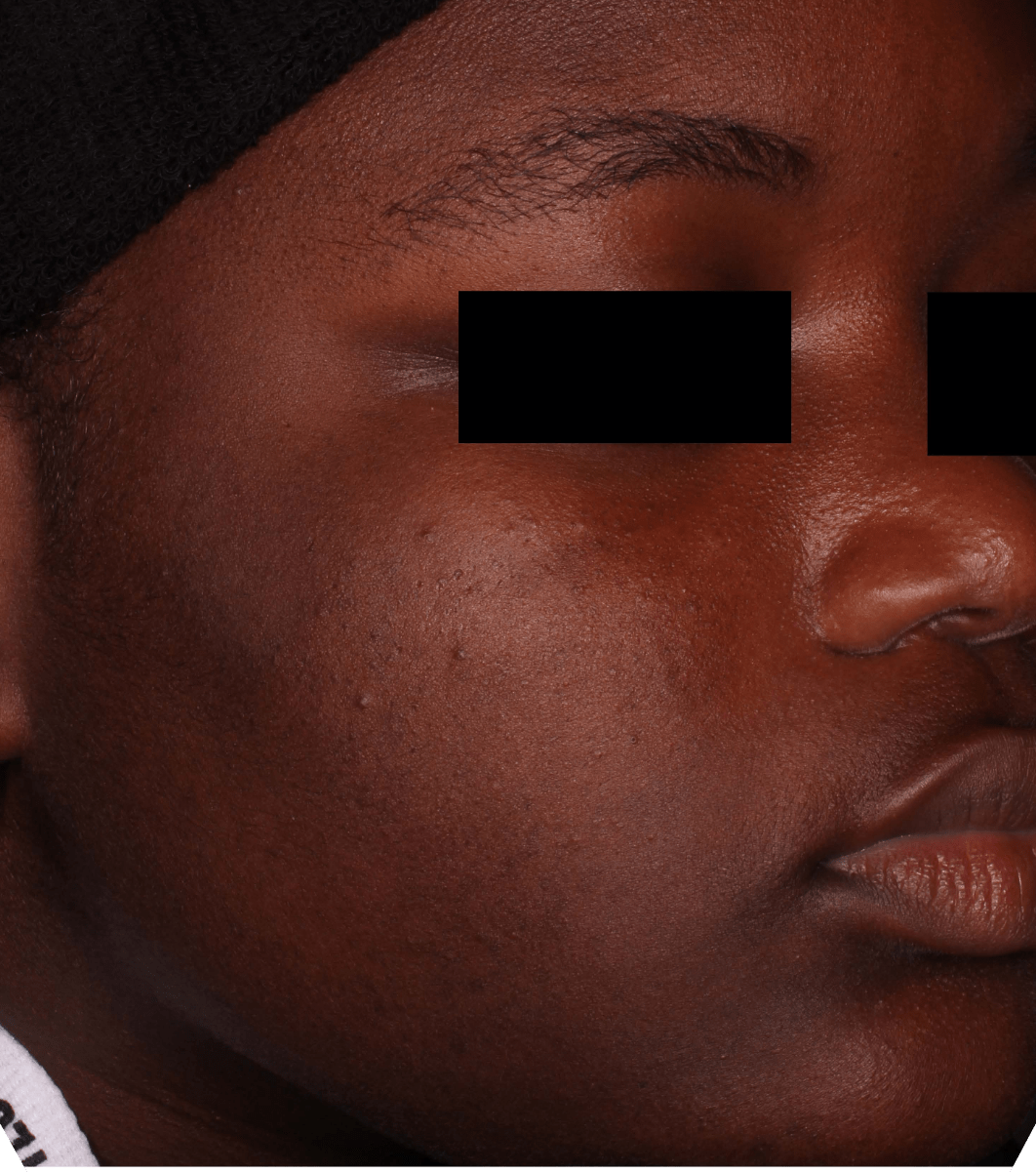
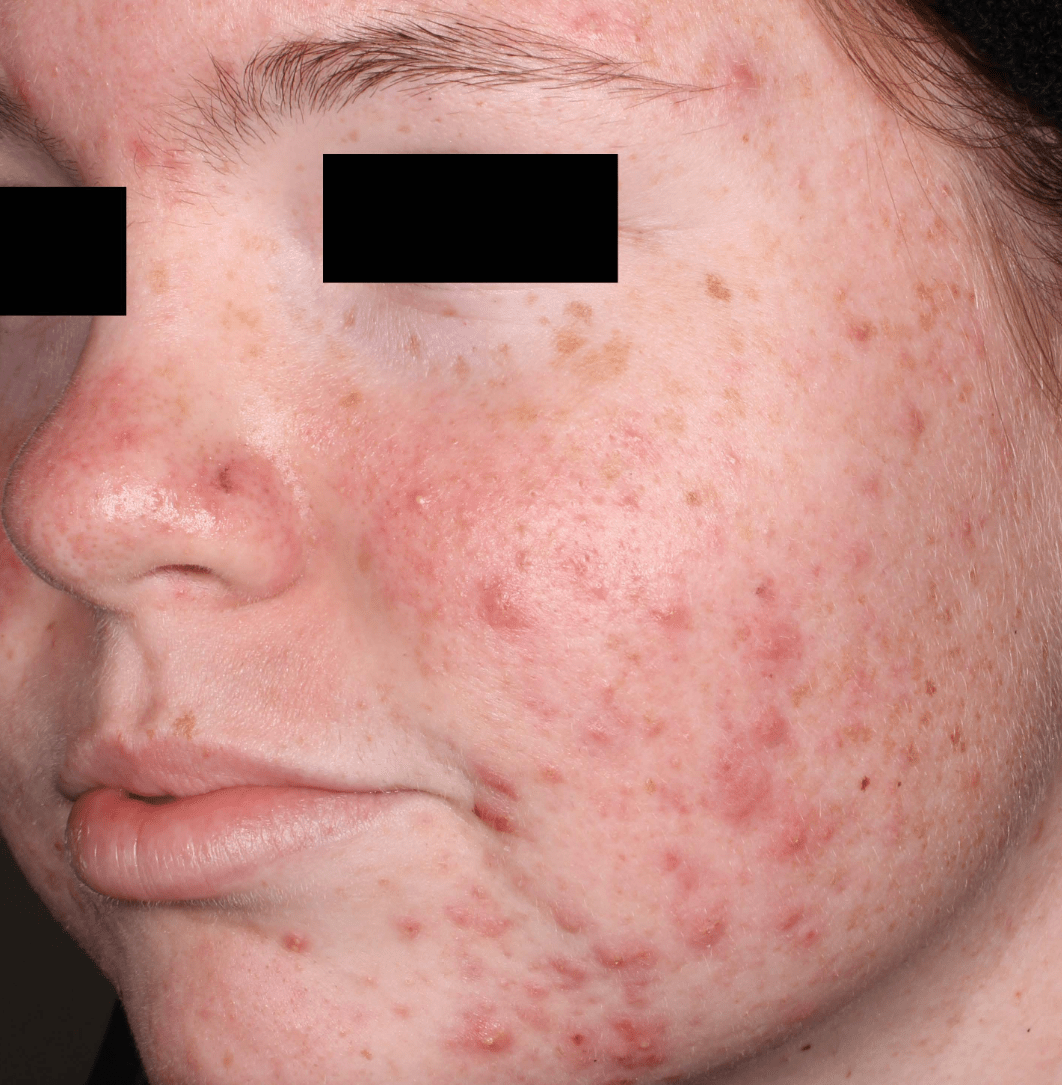
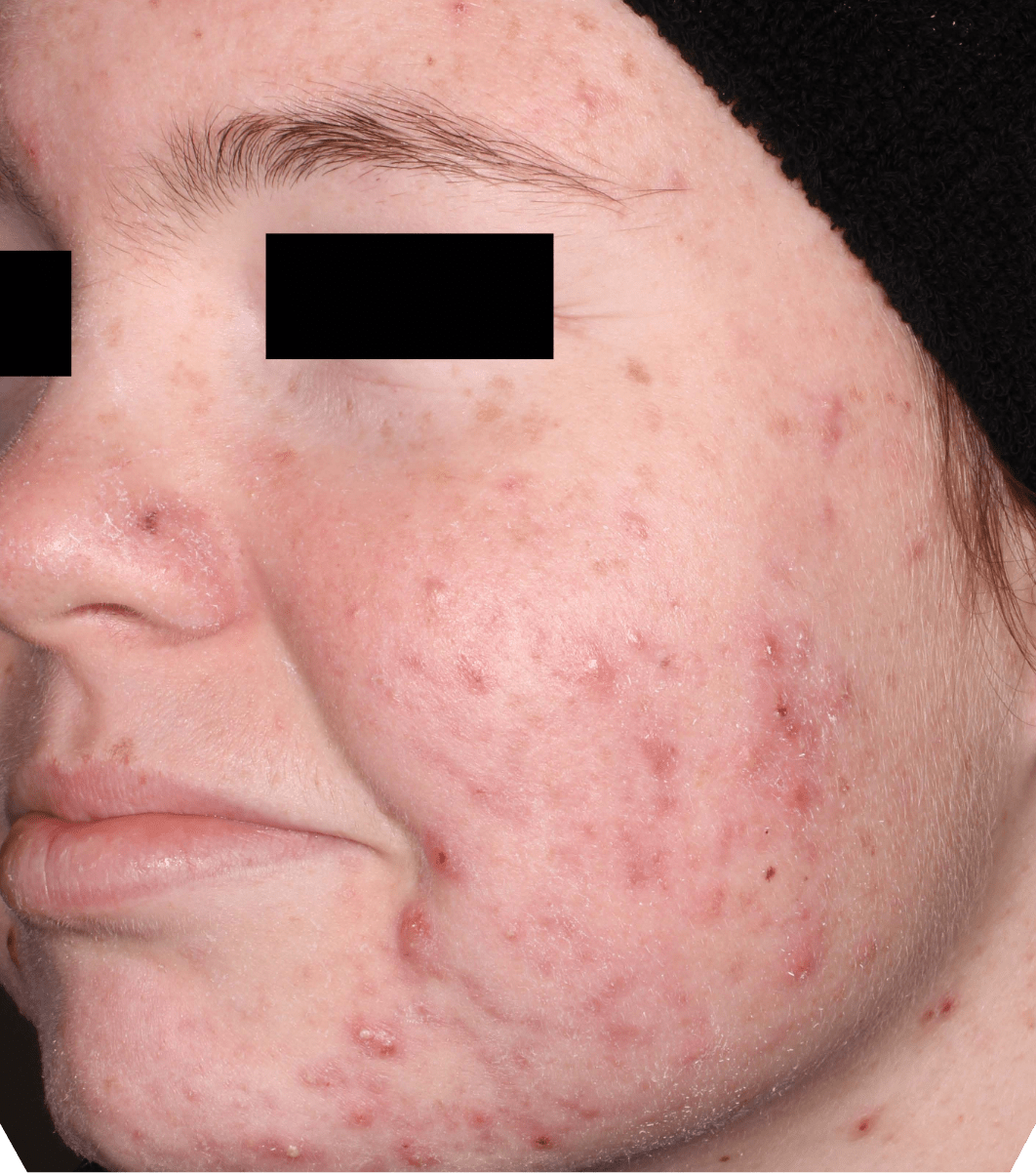
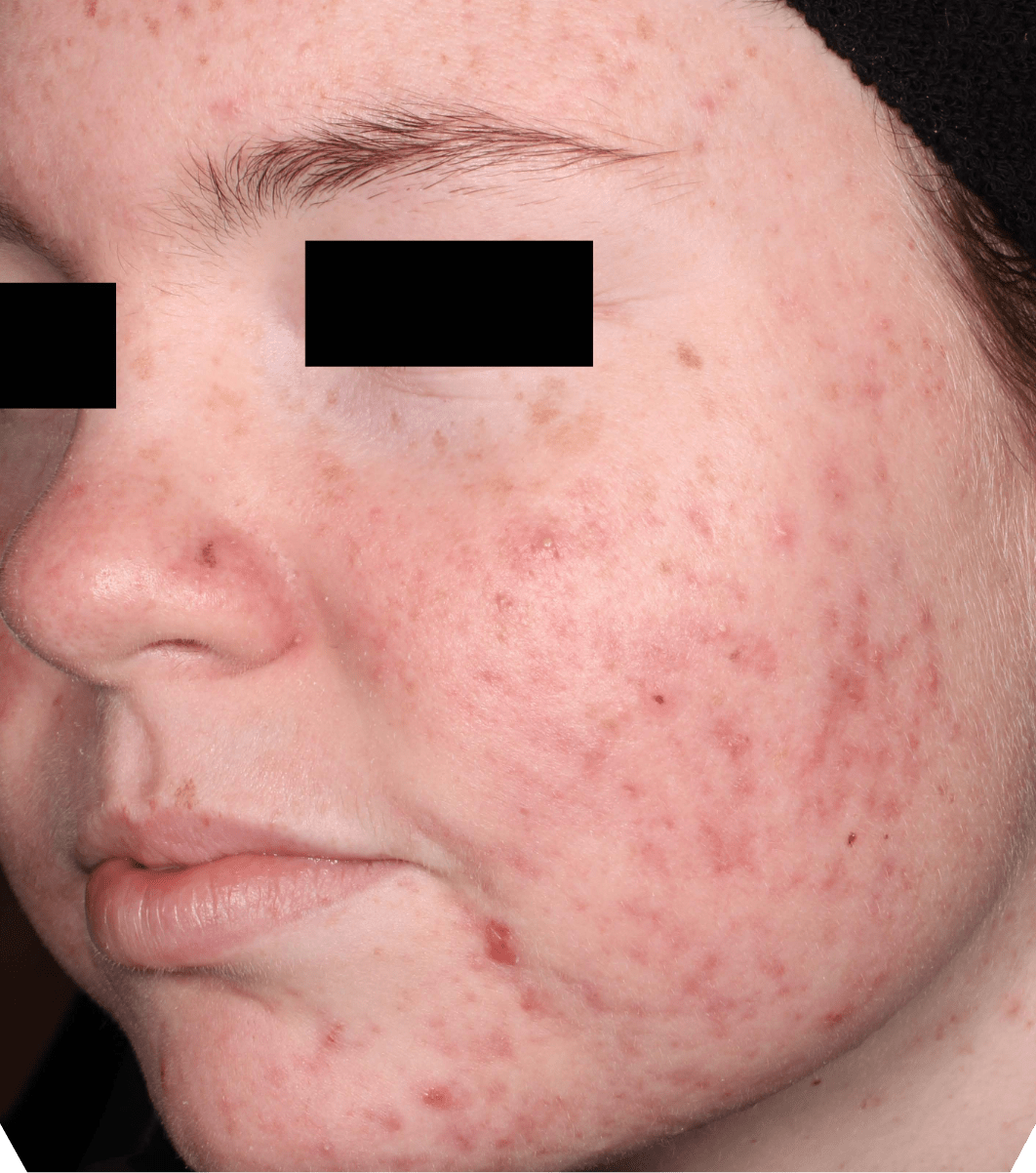
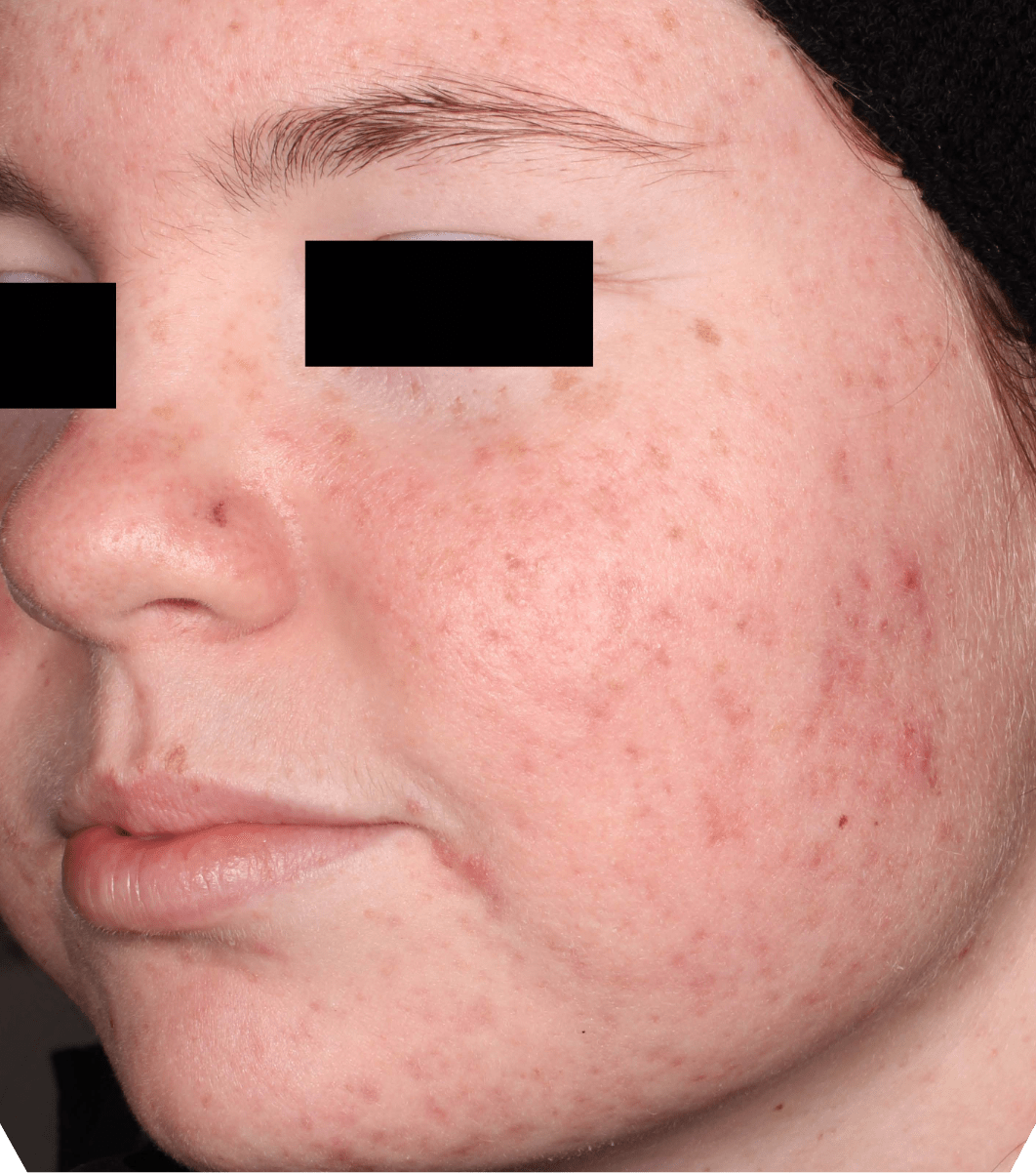
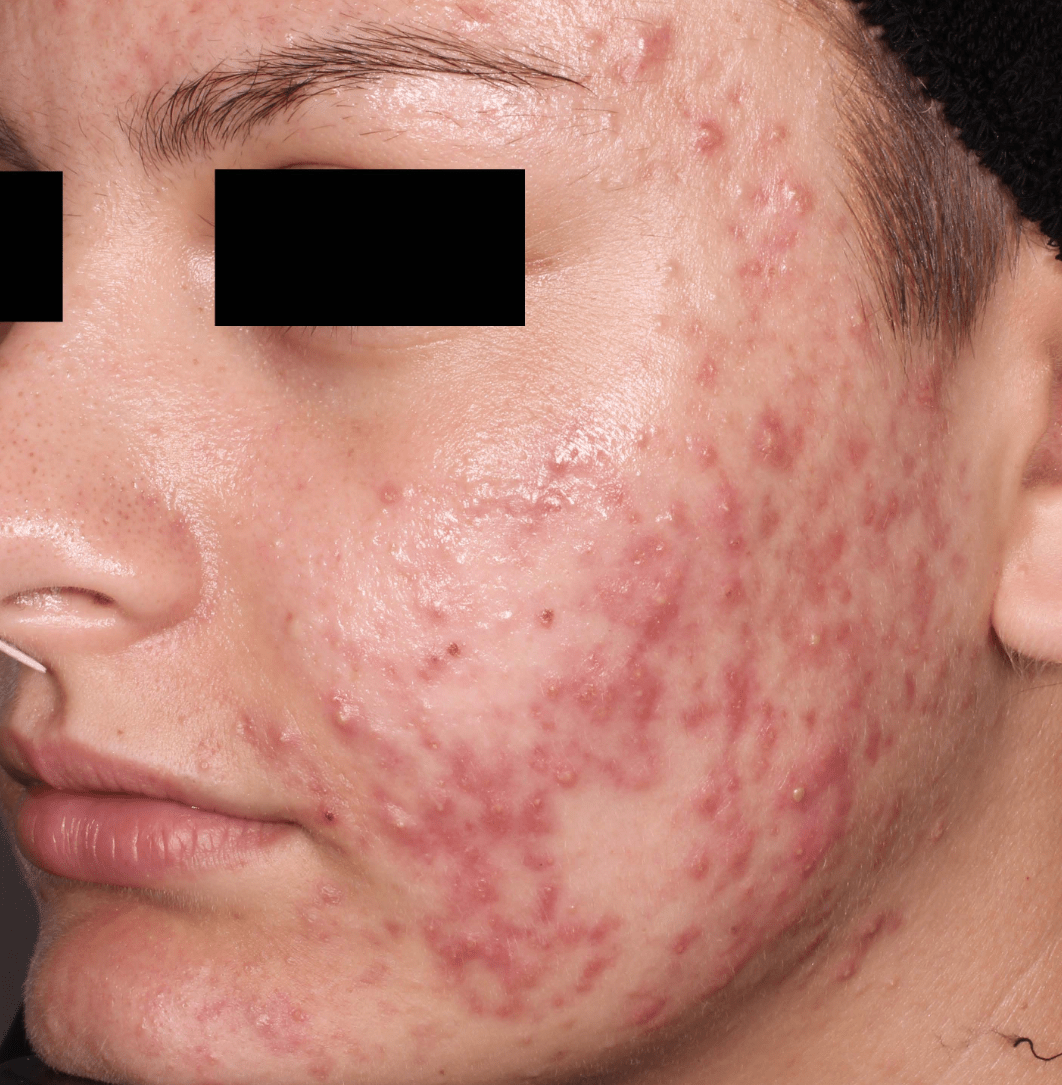
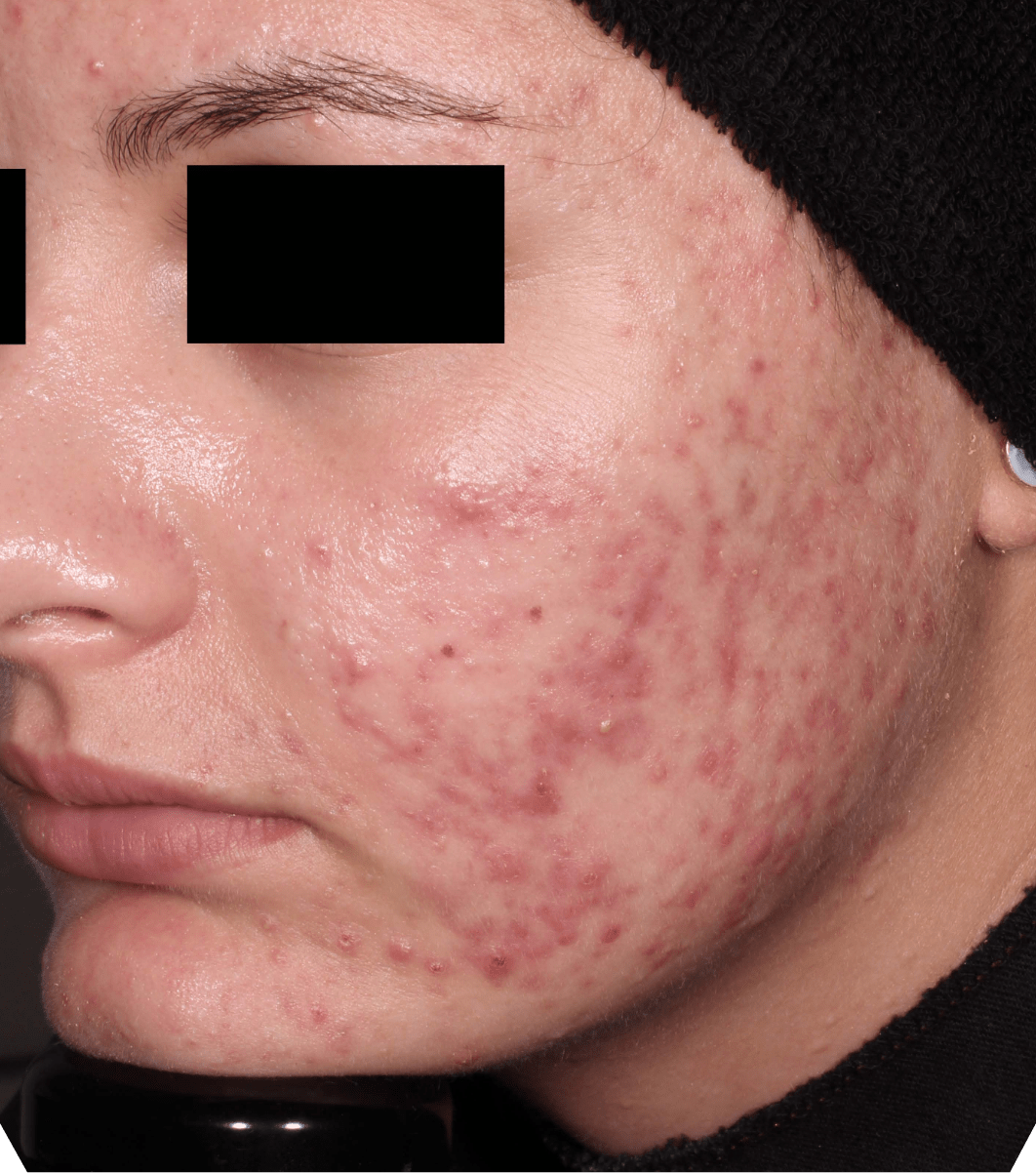
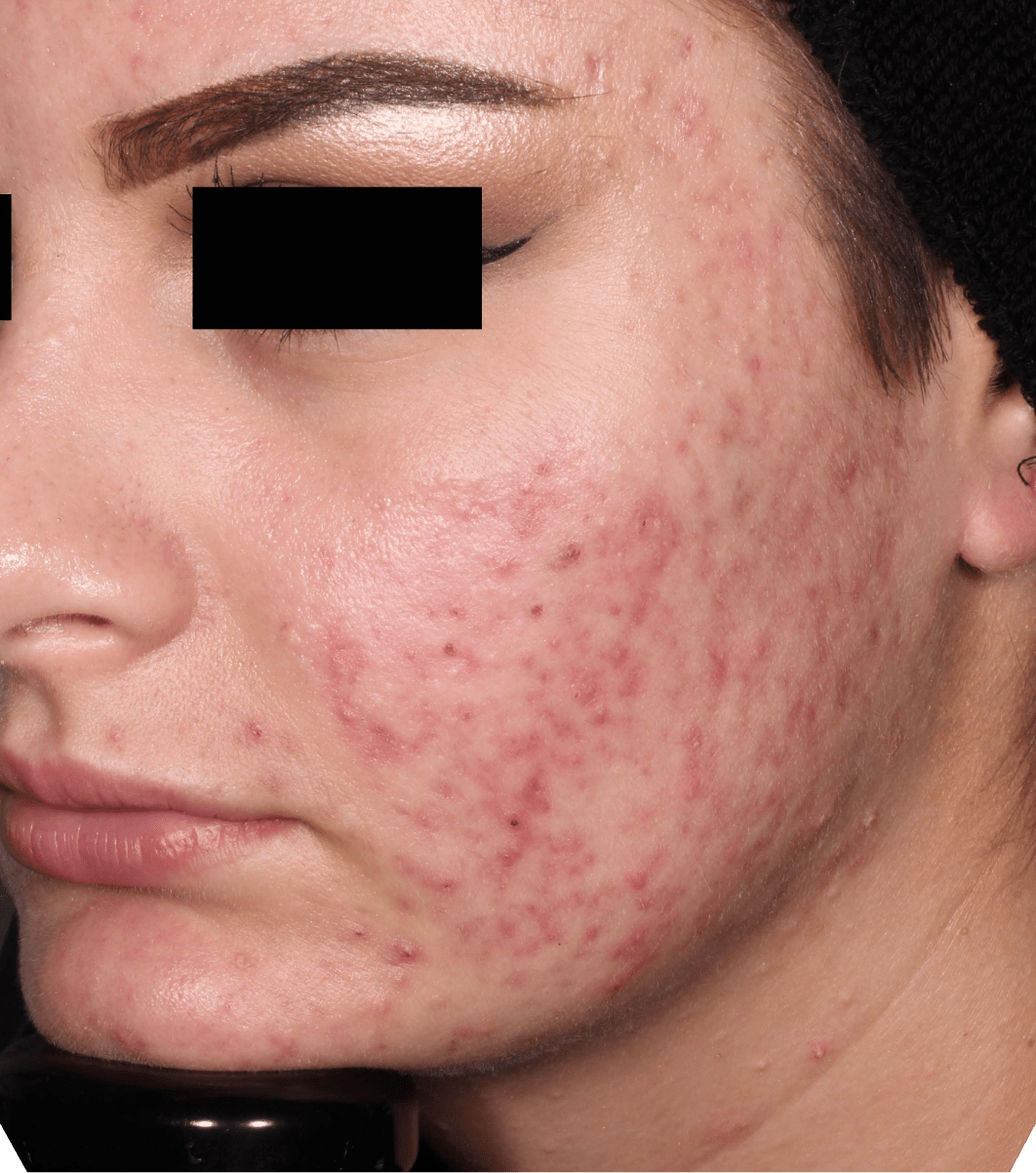
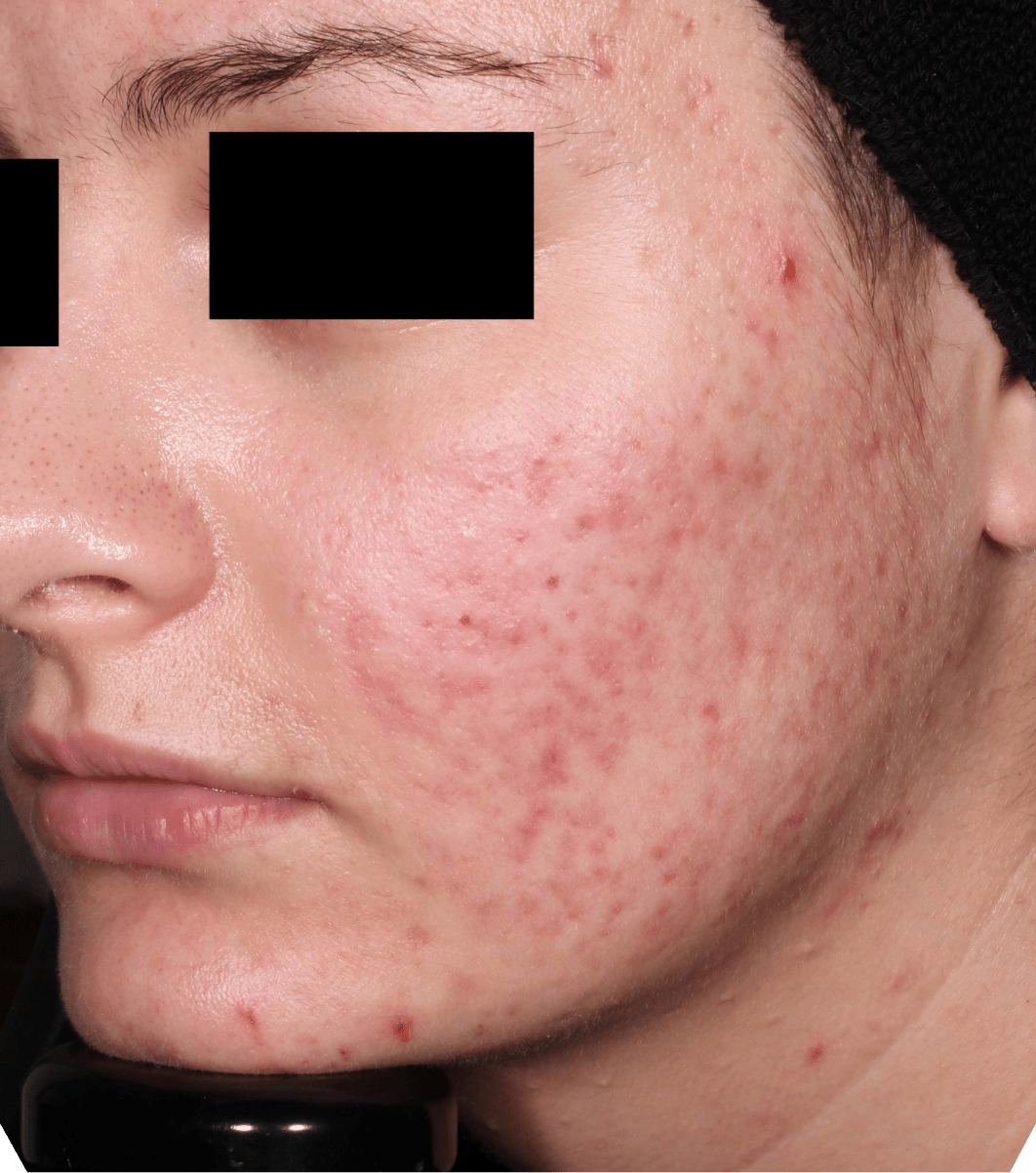
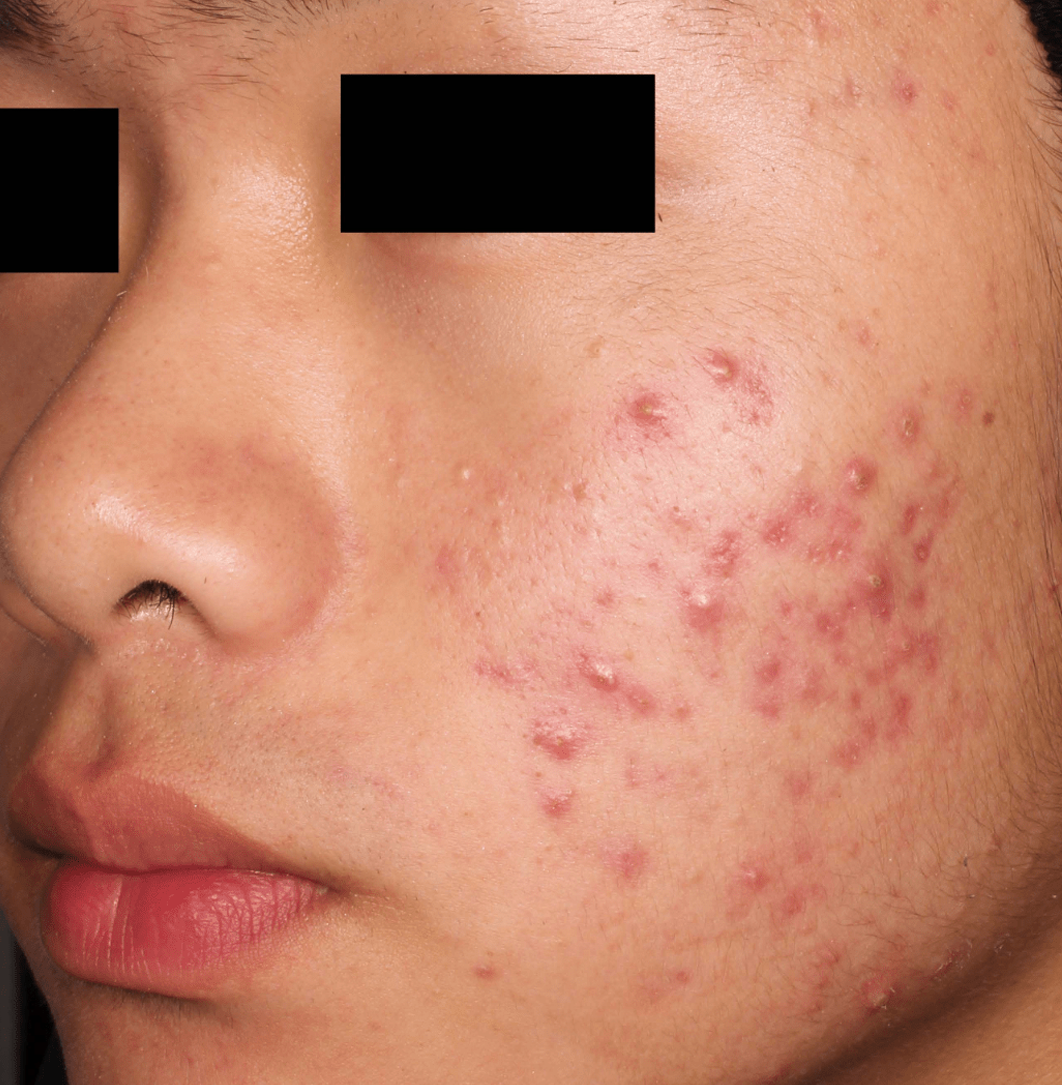
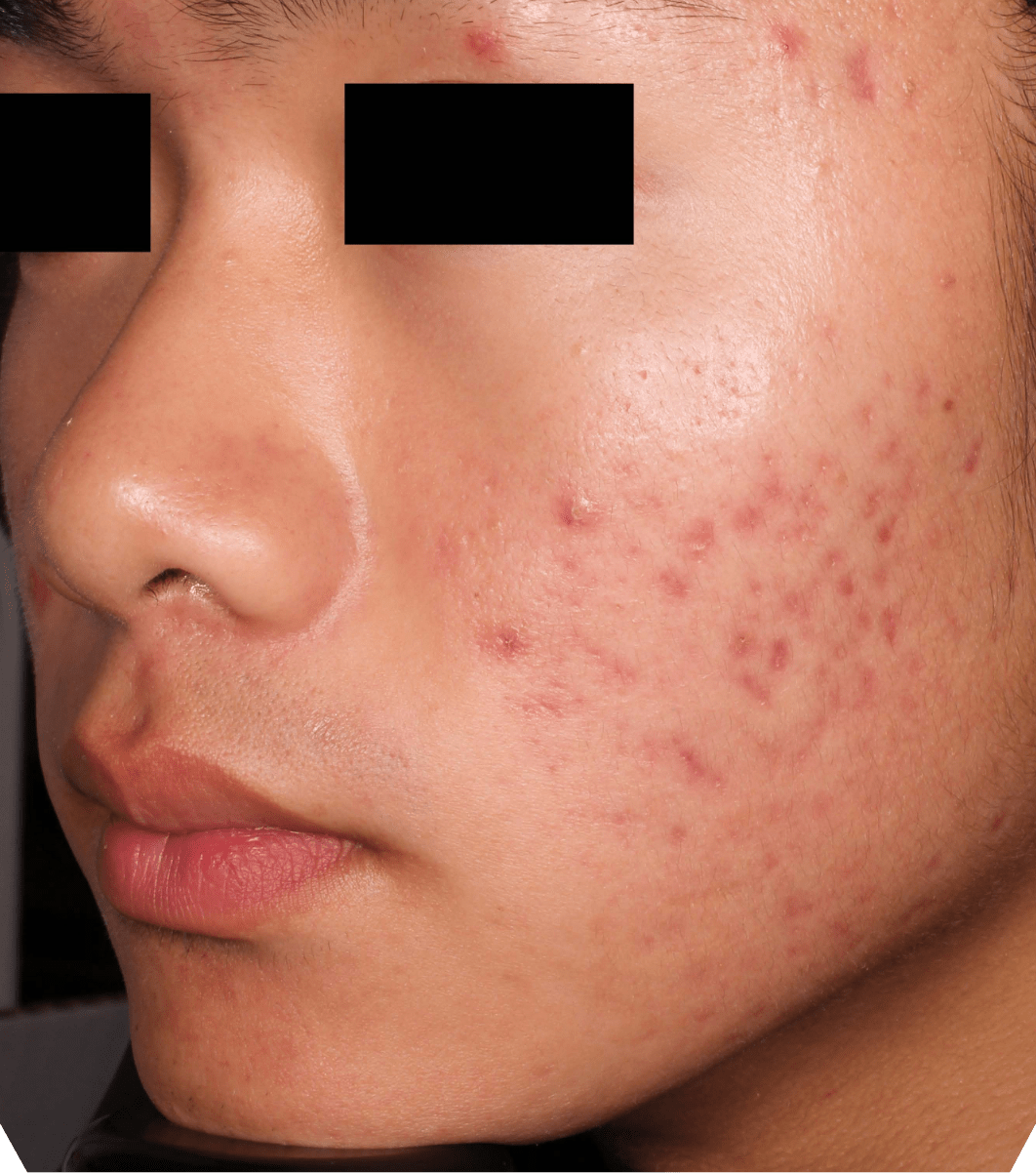
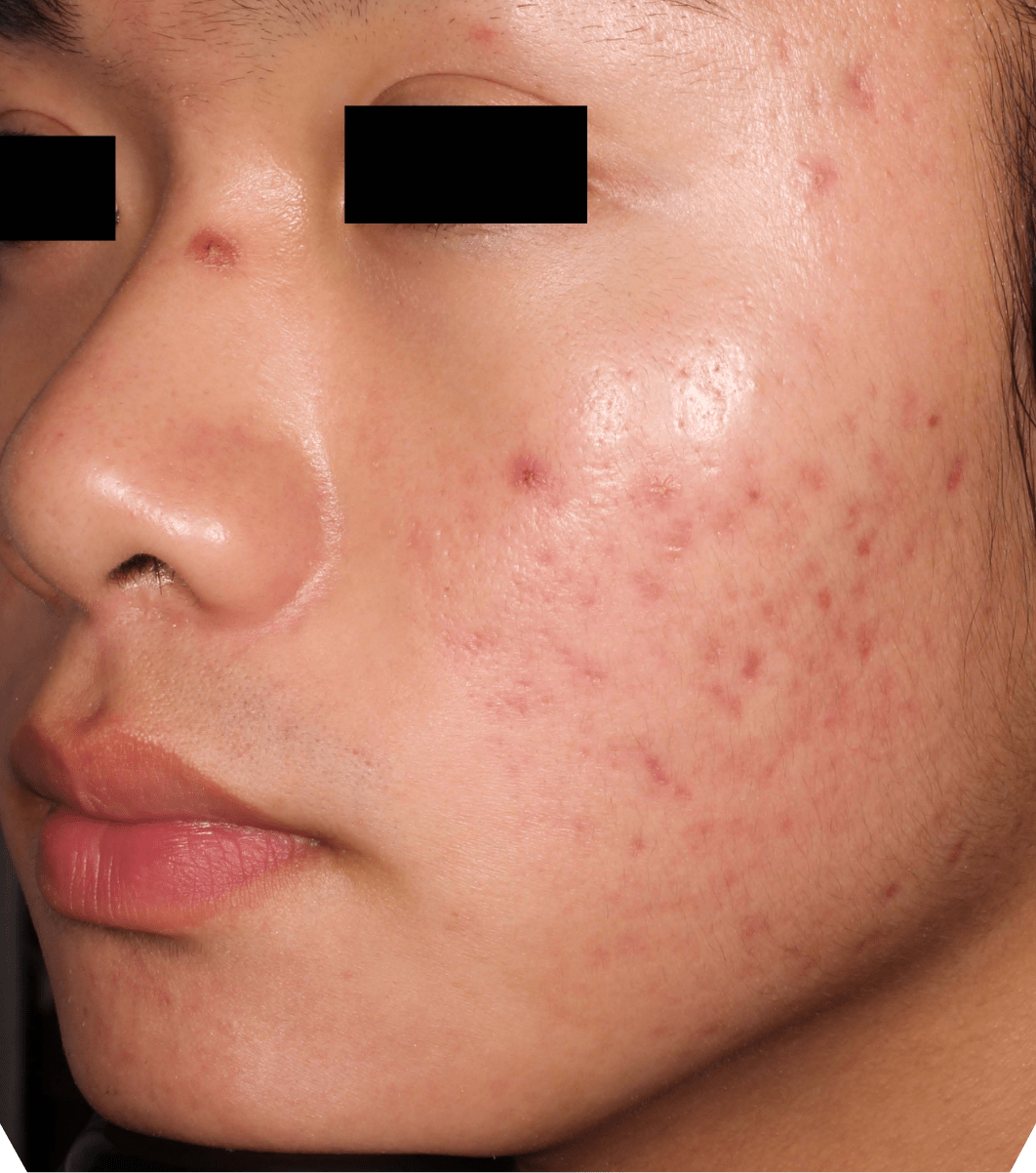
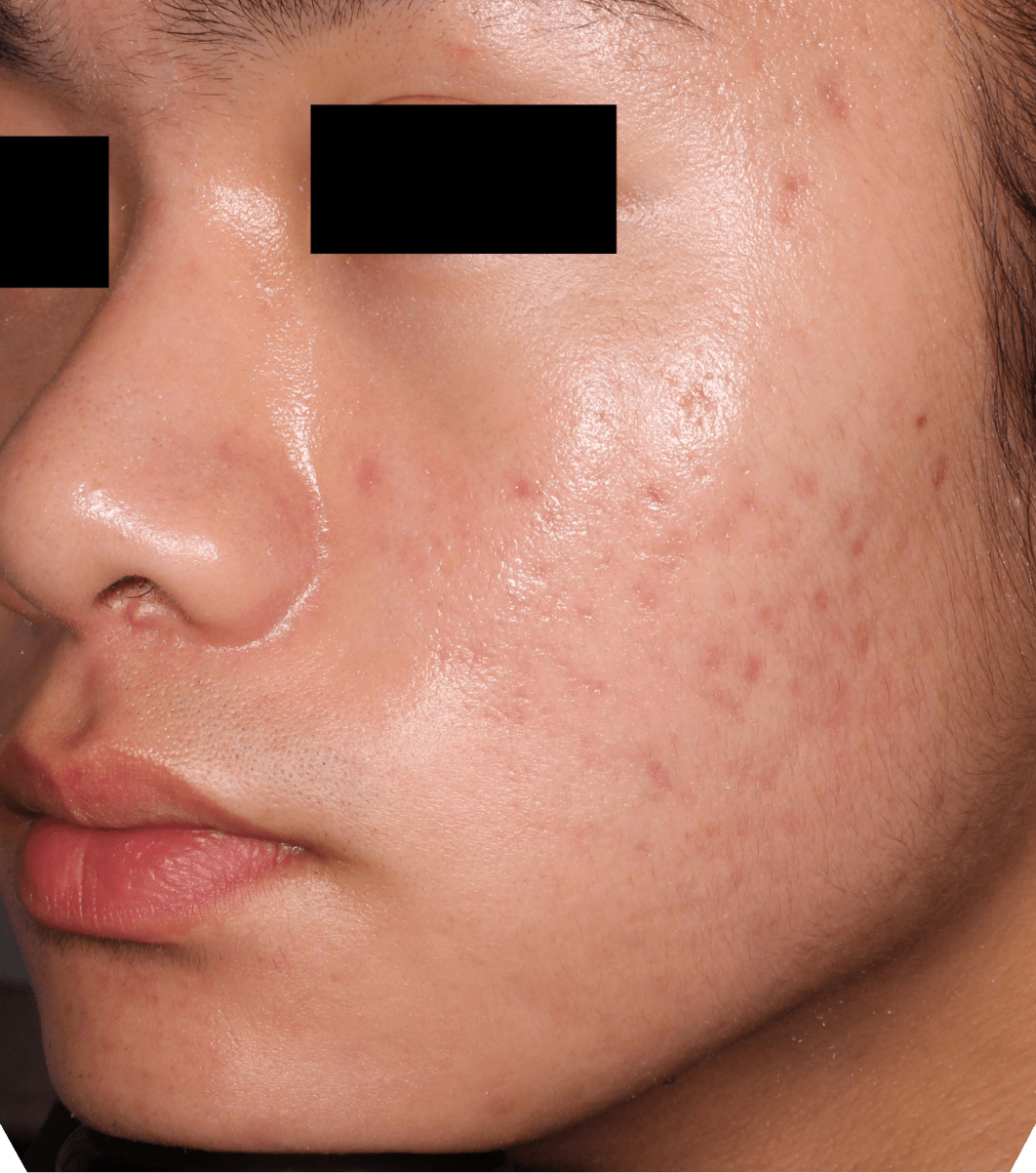
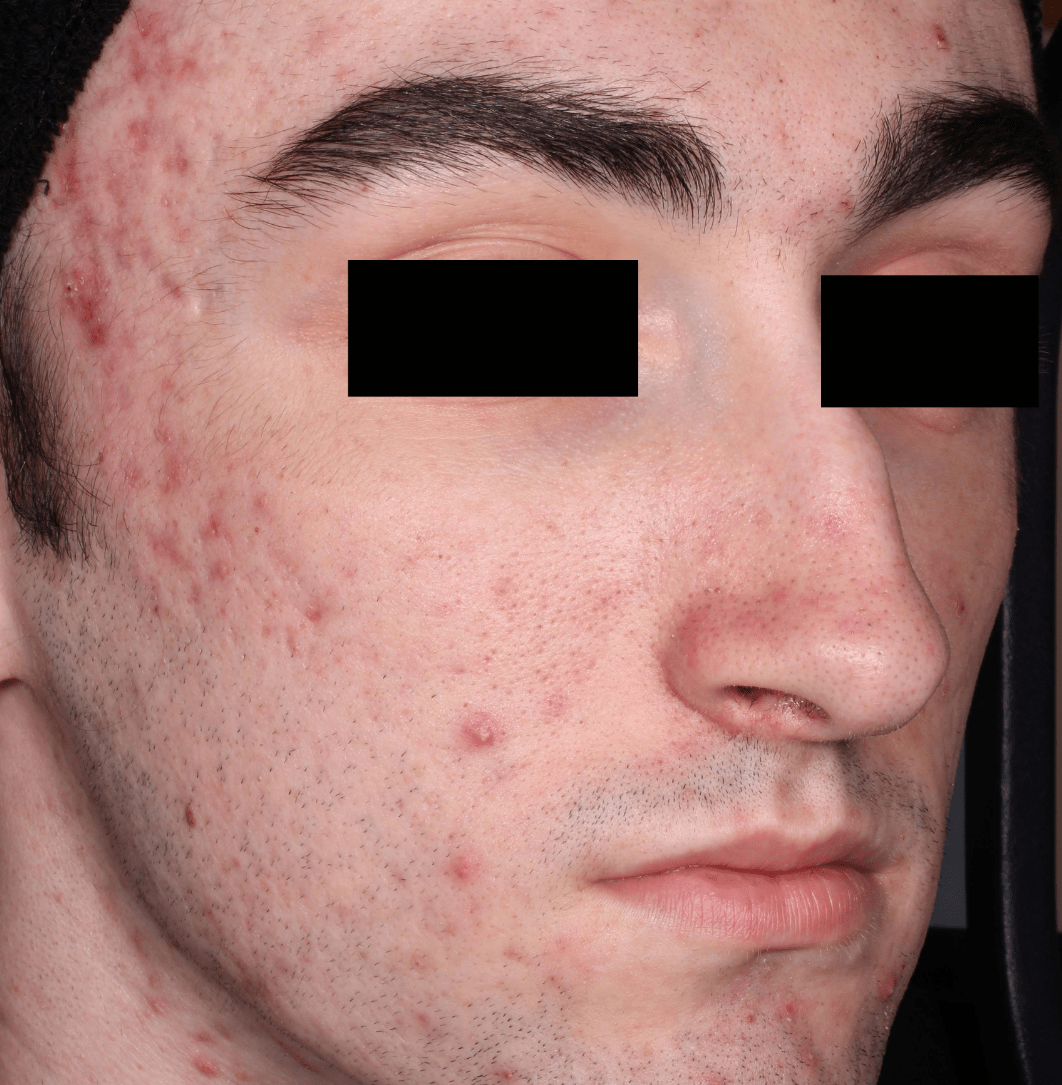
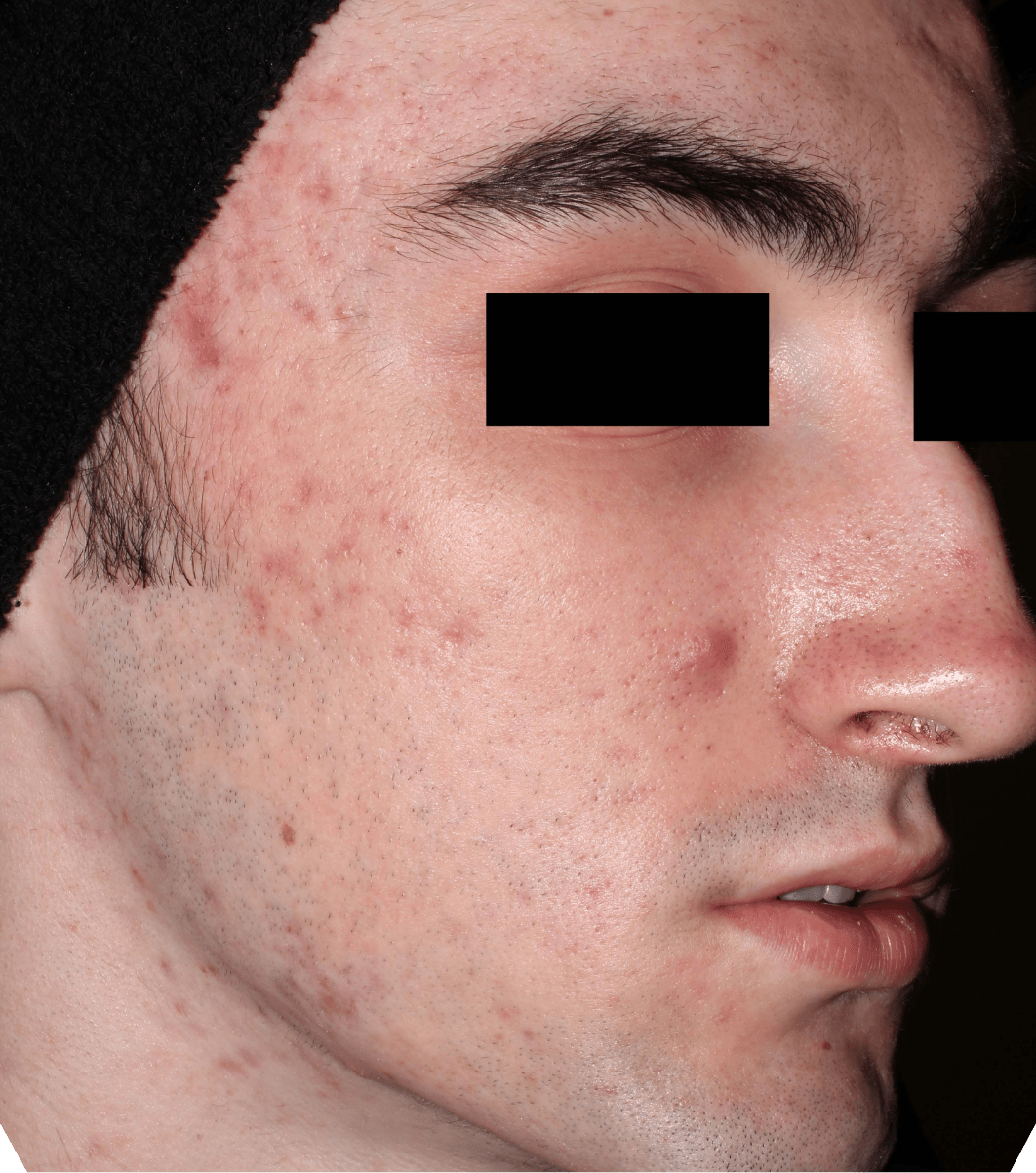
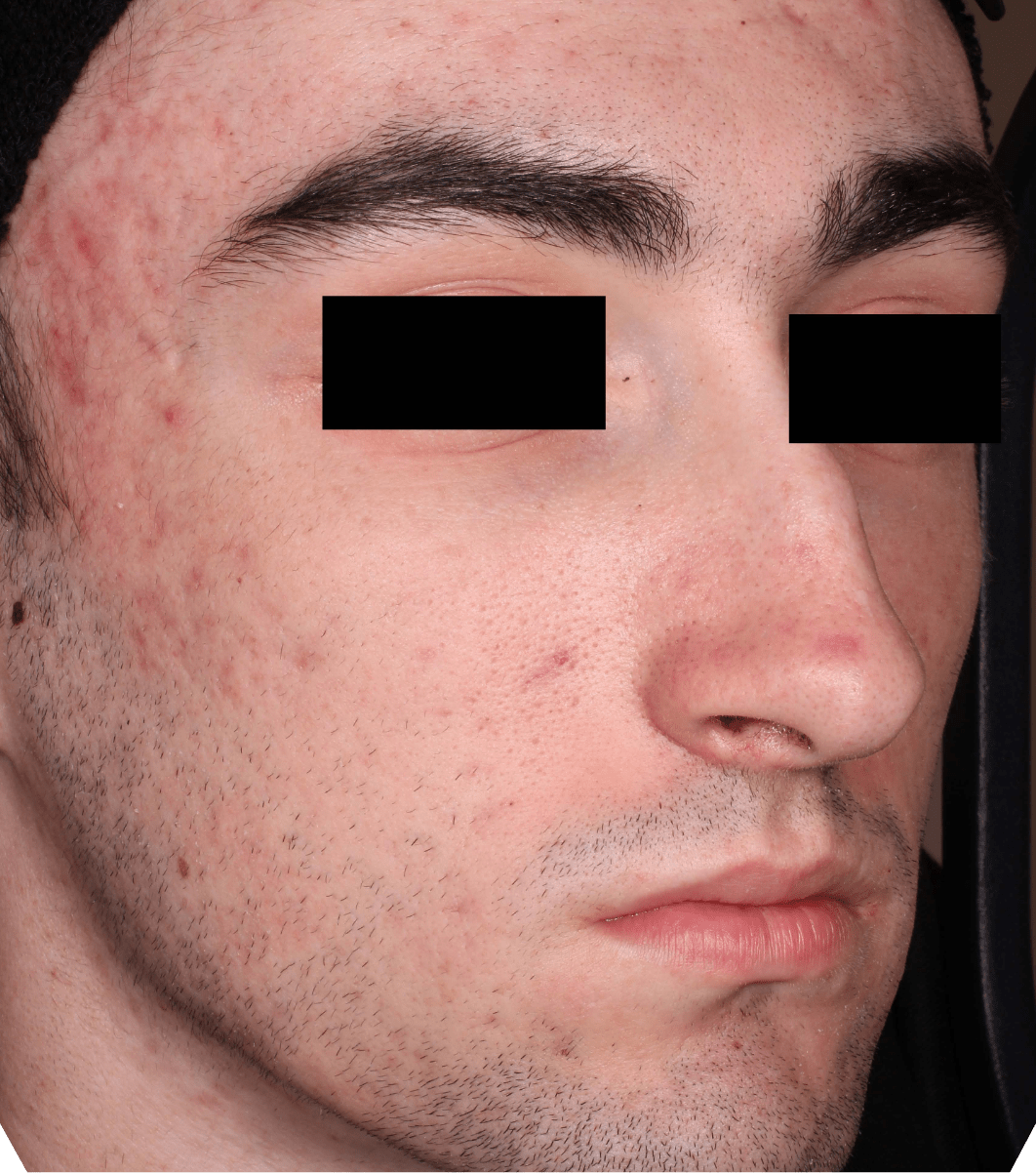
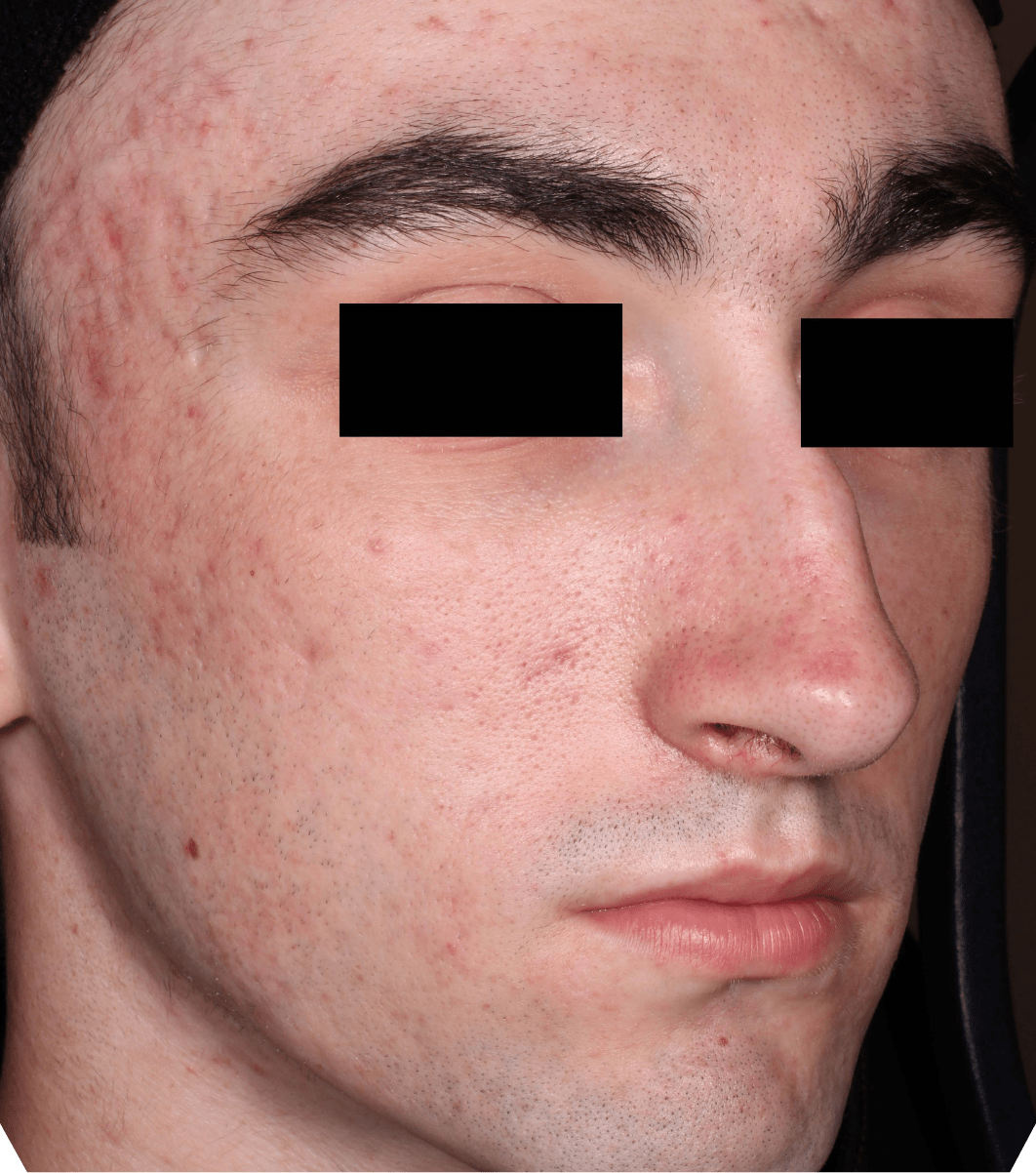
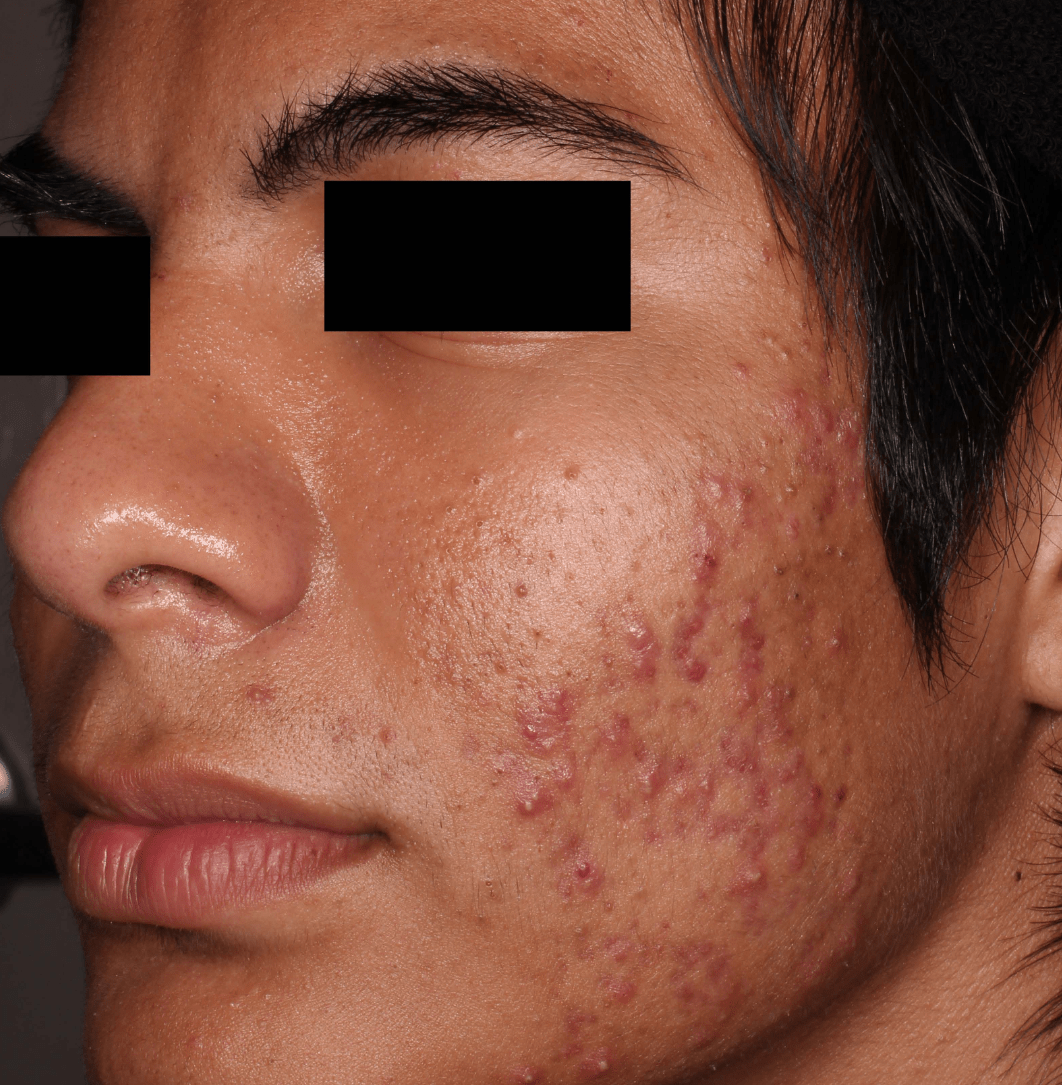
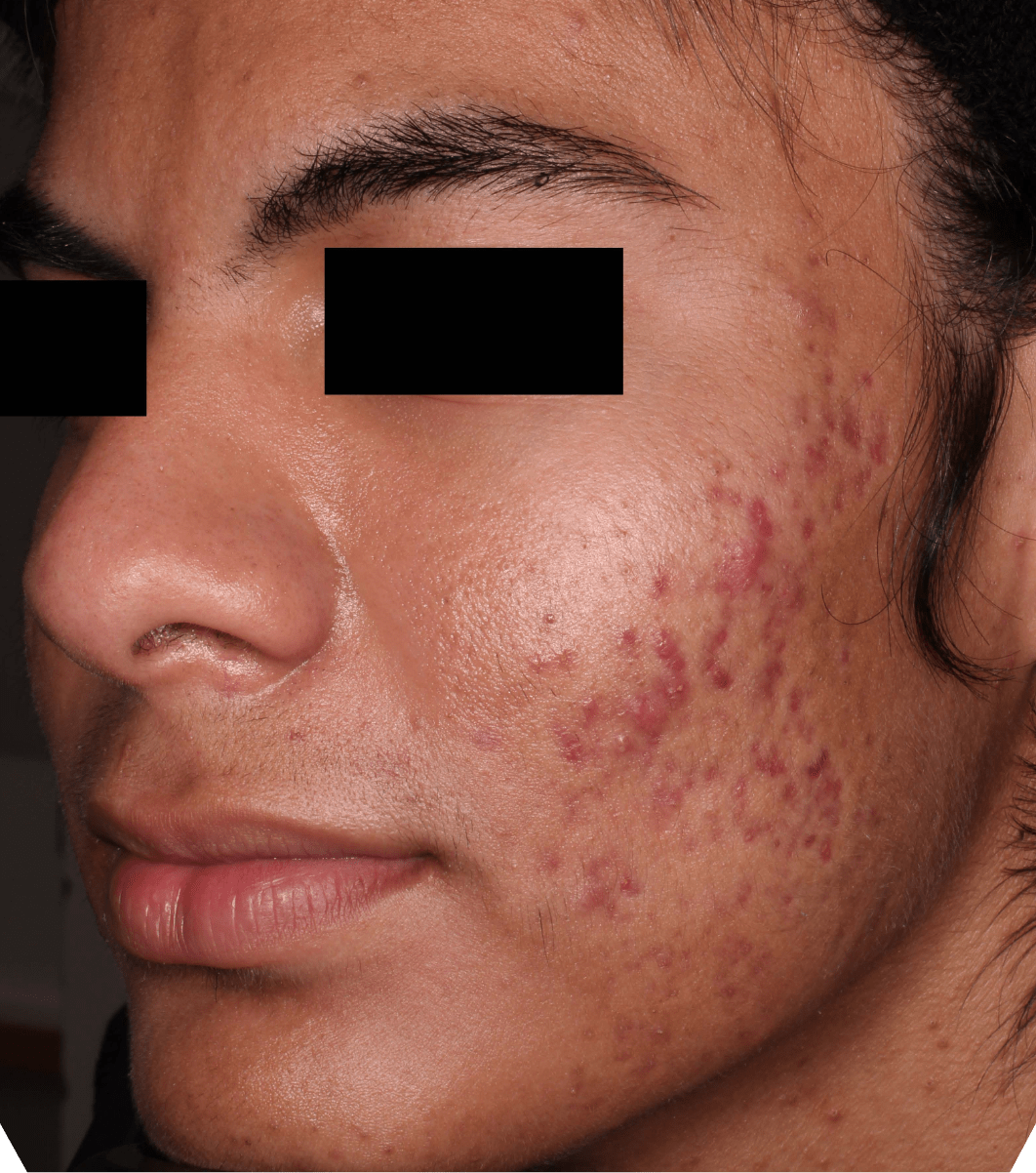
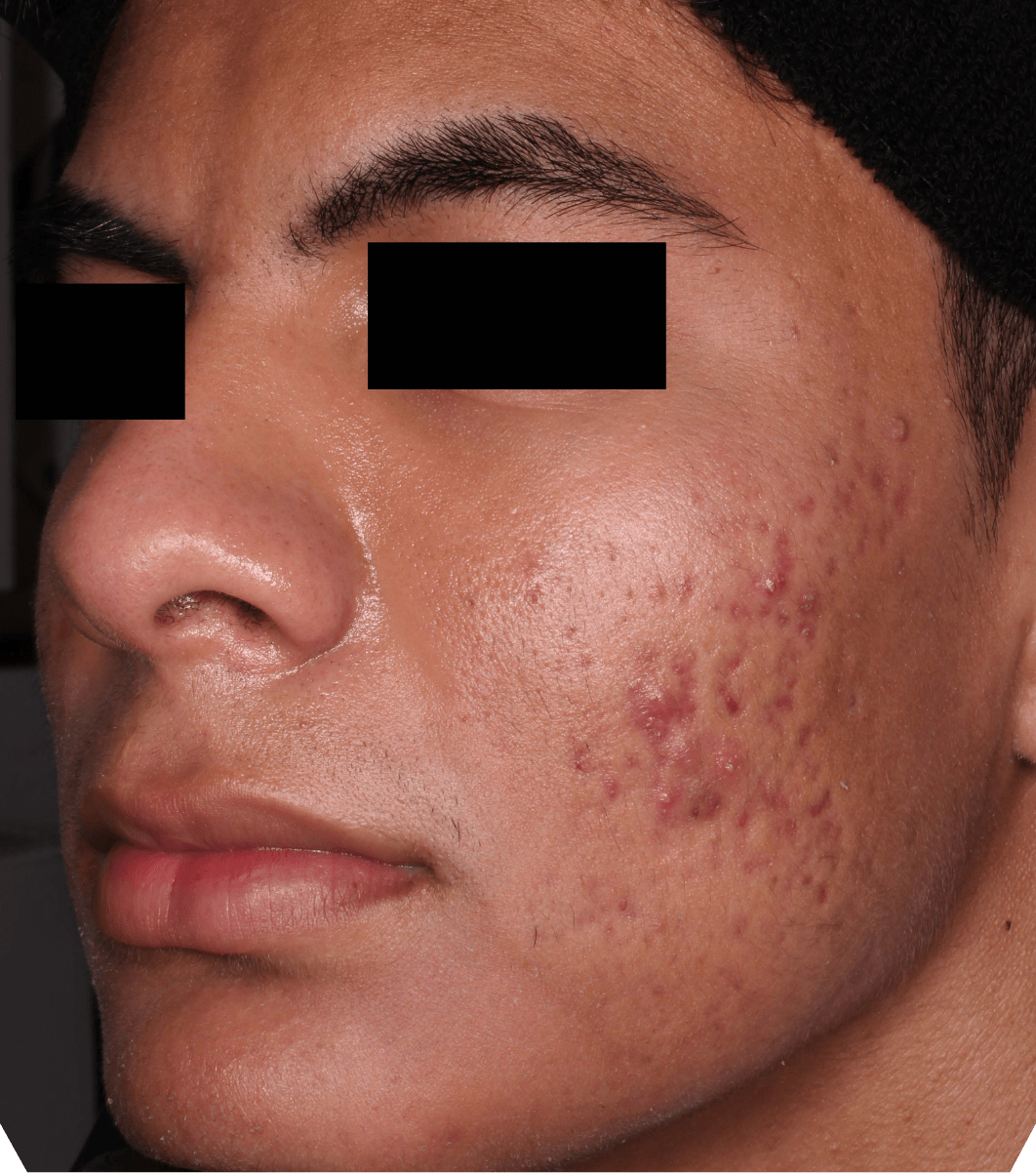
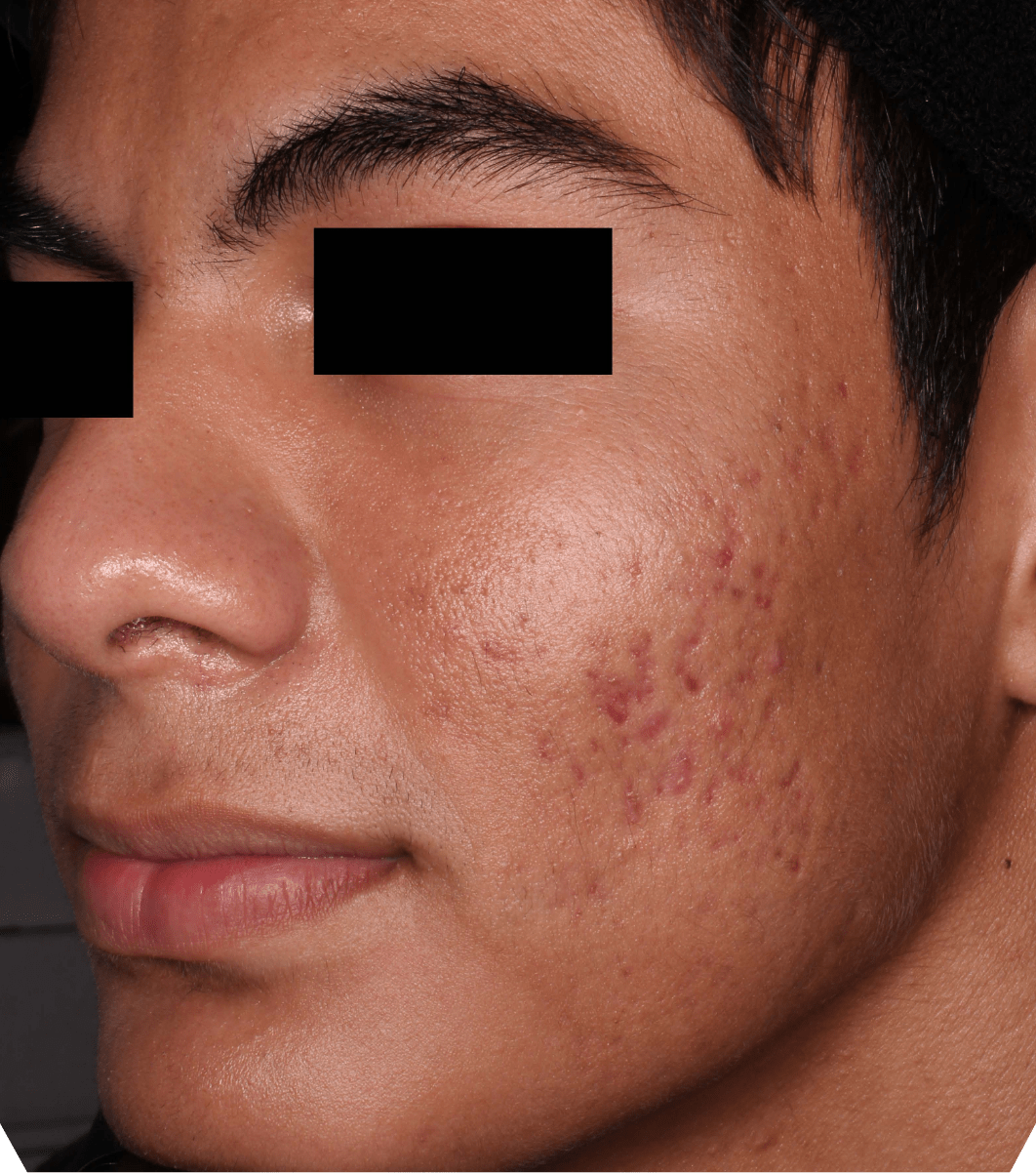
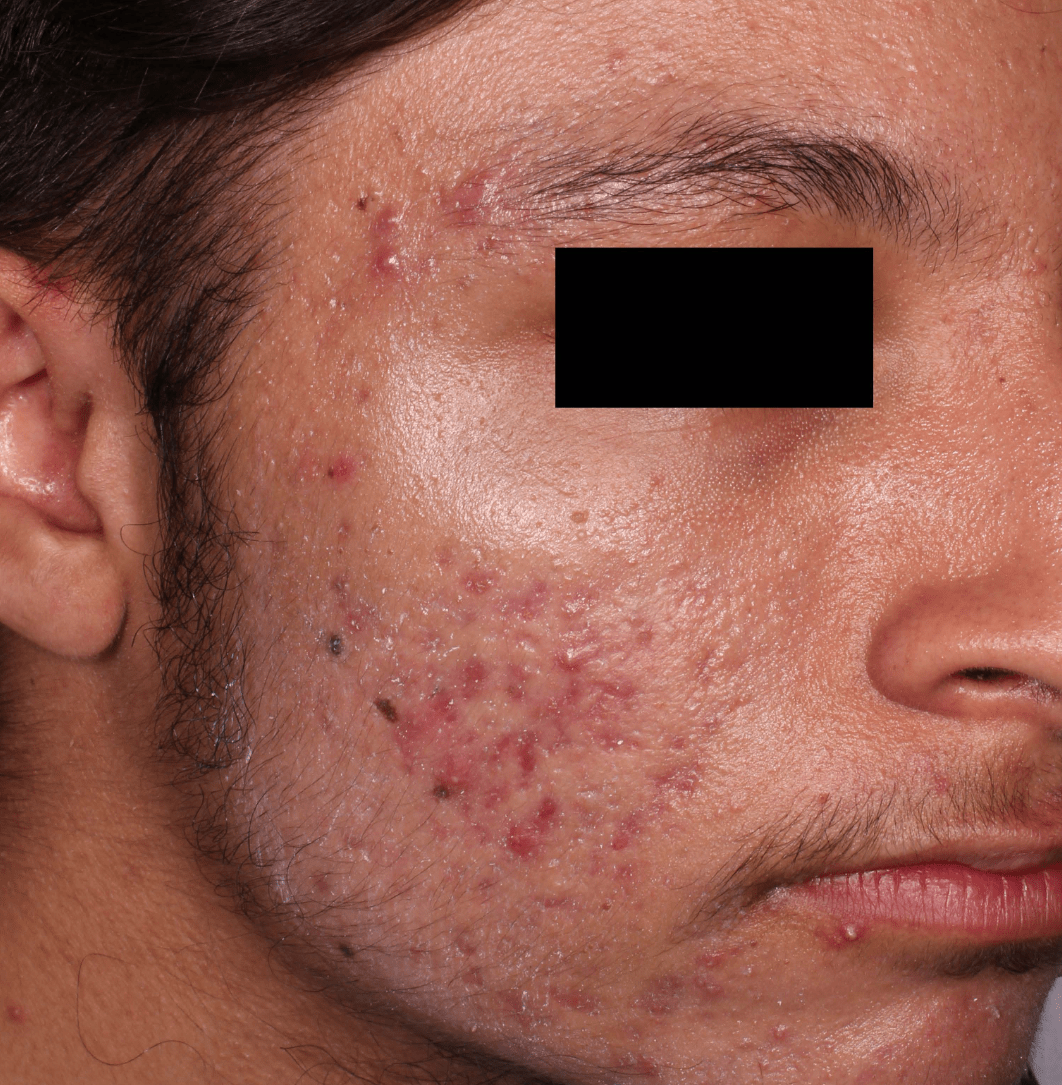
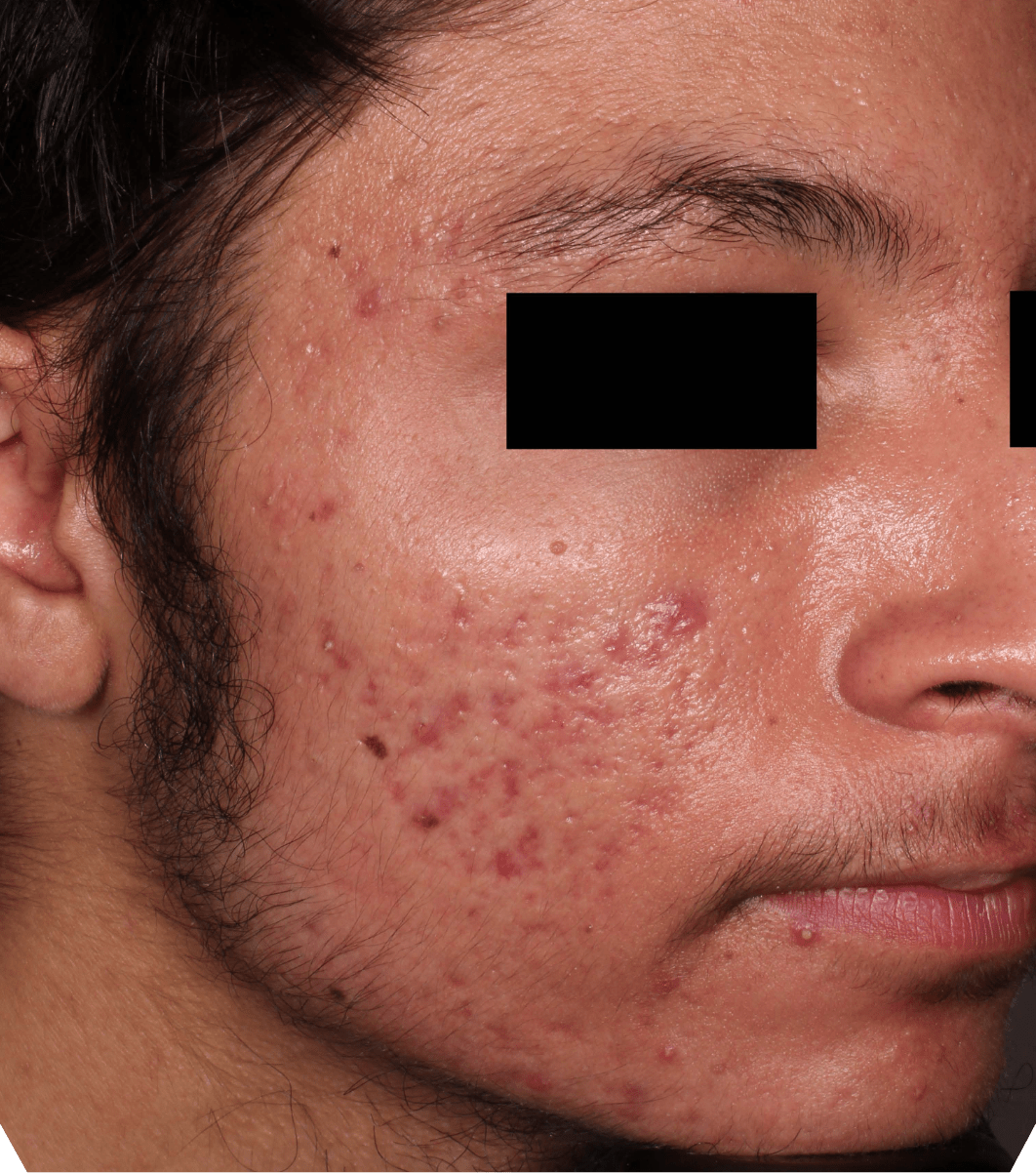
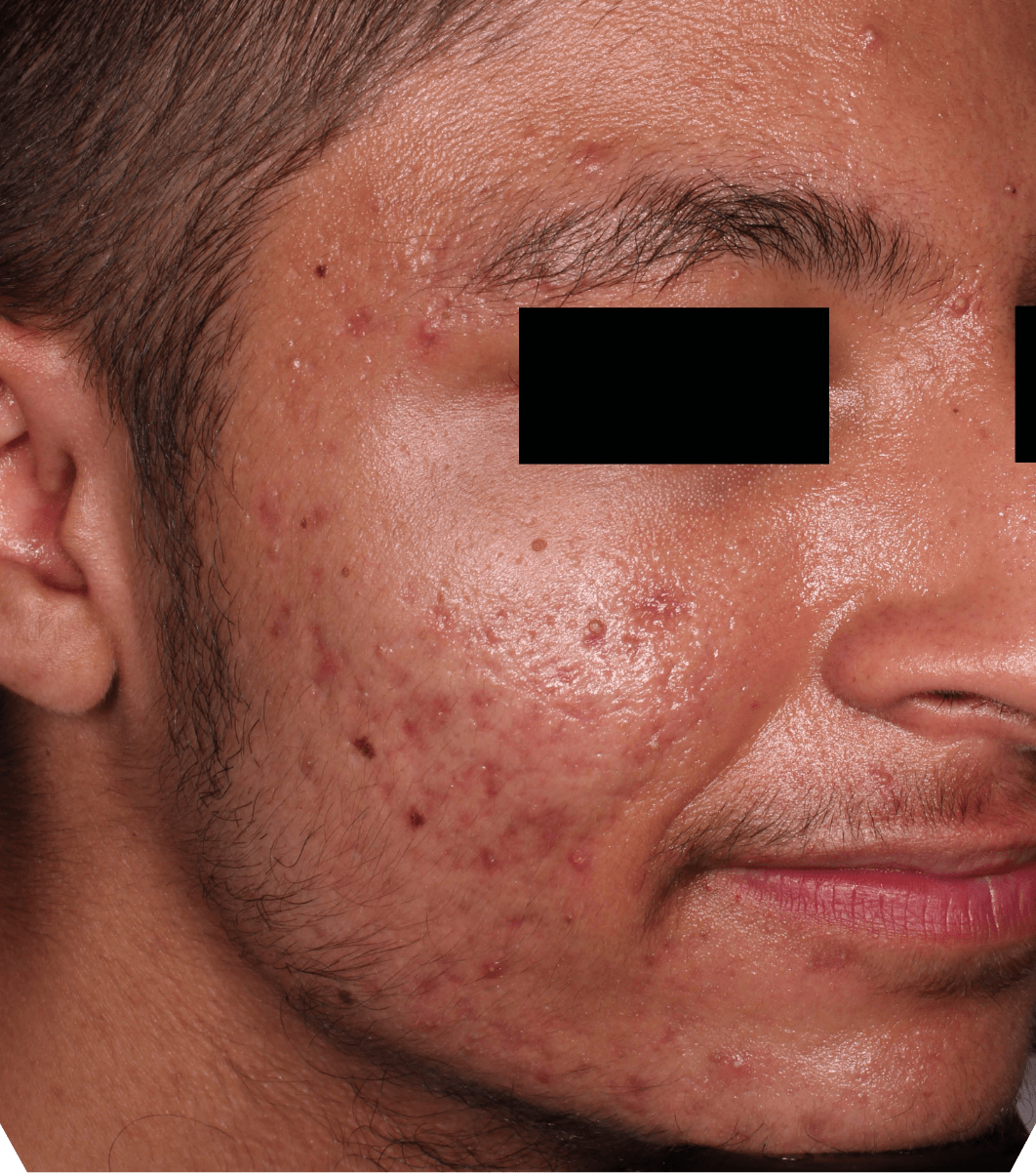
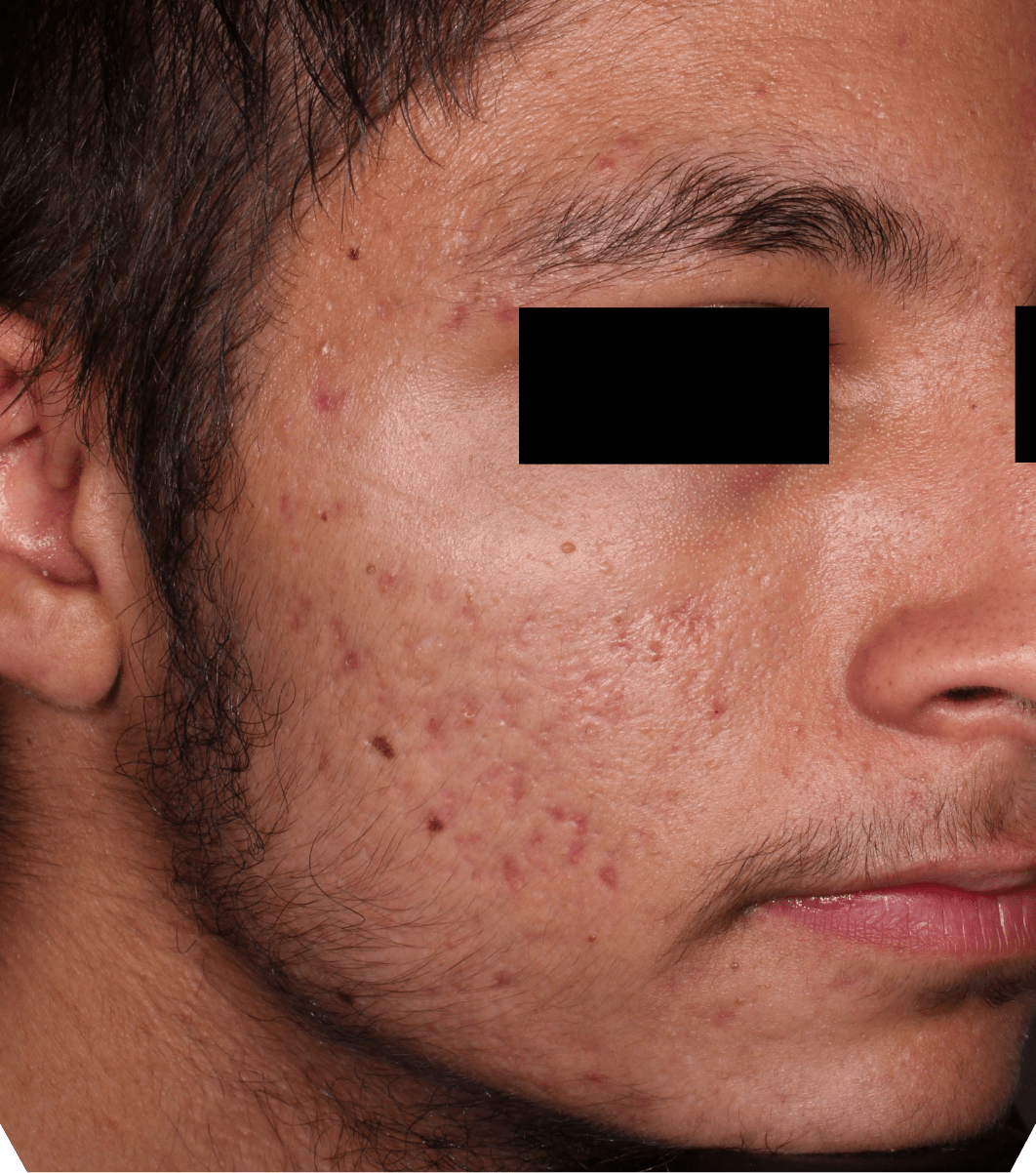
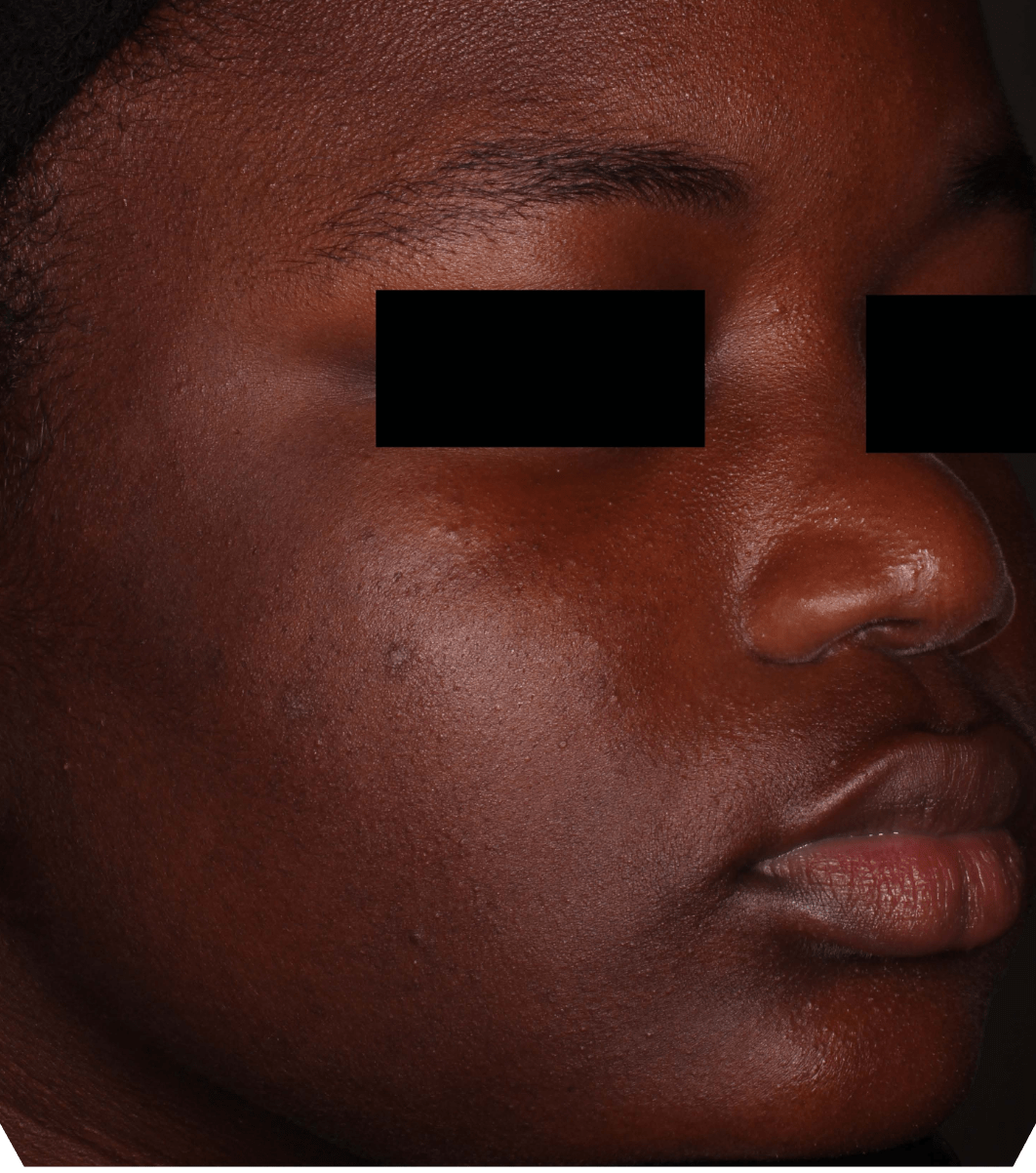Not an actual patient.

Before-and-after photos from clinical trials1,2
Individual results may vary.
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind multicenter, randomized, 12-week study of 741 subjects ≥9 years of age and older with facial acne vulgaris3
- Subjects were equally randomized to once-daily CABTREO, vehicle, or 1 of 3 component dyad gels: BPO 3.1%/adapalene 0.15%; clindamycin phosphate 1.2%/BPO 3.1%; or clindamycin phosphate 1.2%/adapalene 0.15%3
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules3
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety tolerability were also assessed3
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind multicenter, randomized, 12-week study of 741 subjects ≥9 years of age and older with facial acne vulgaris3
- Subjects were equally randomized to once-daily CABTREO, vehicle, or 1 of 3 component dyad gels: BPO 3.1%/adapalene 0.15%; clindamycin phosphate 1.2%/BPO 3.1%; or clindamycin phosphate 1.2%/adapalene 0.15%3
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules3
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety tolerability were also assessed3
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle- controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle- controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1


Not an actual patient.
Could CABTREO make a difference in YOUR practice?
A closer look at the diverse patient demographics1,2
Across both Phase 3 trials, patients were1,2:
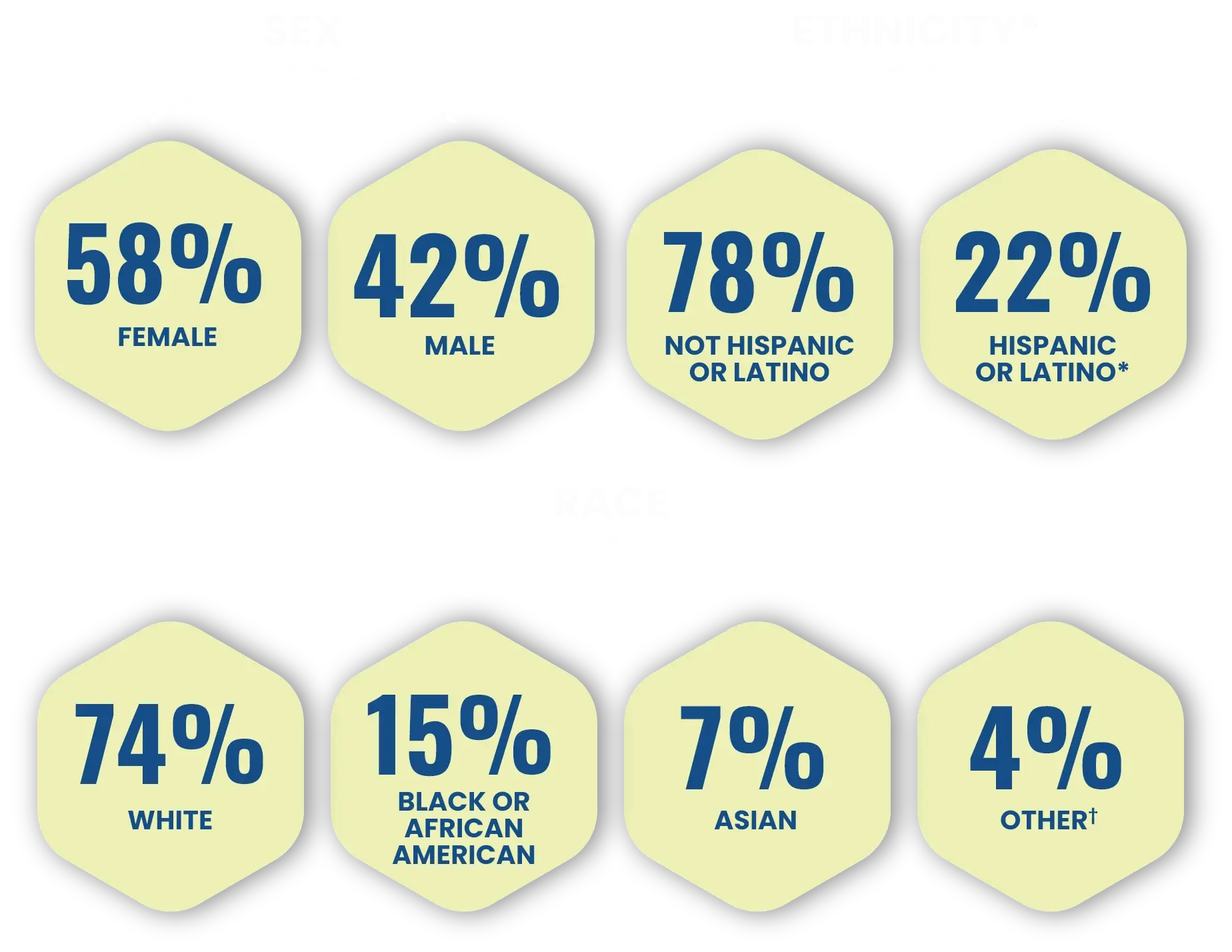
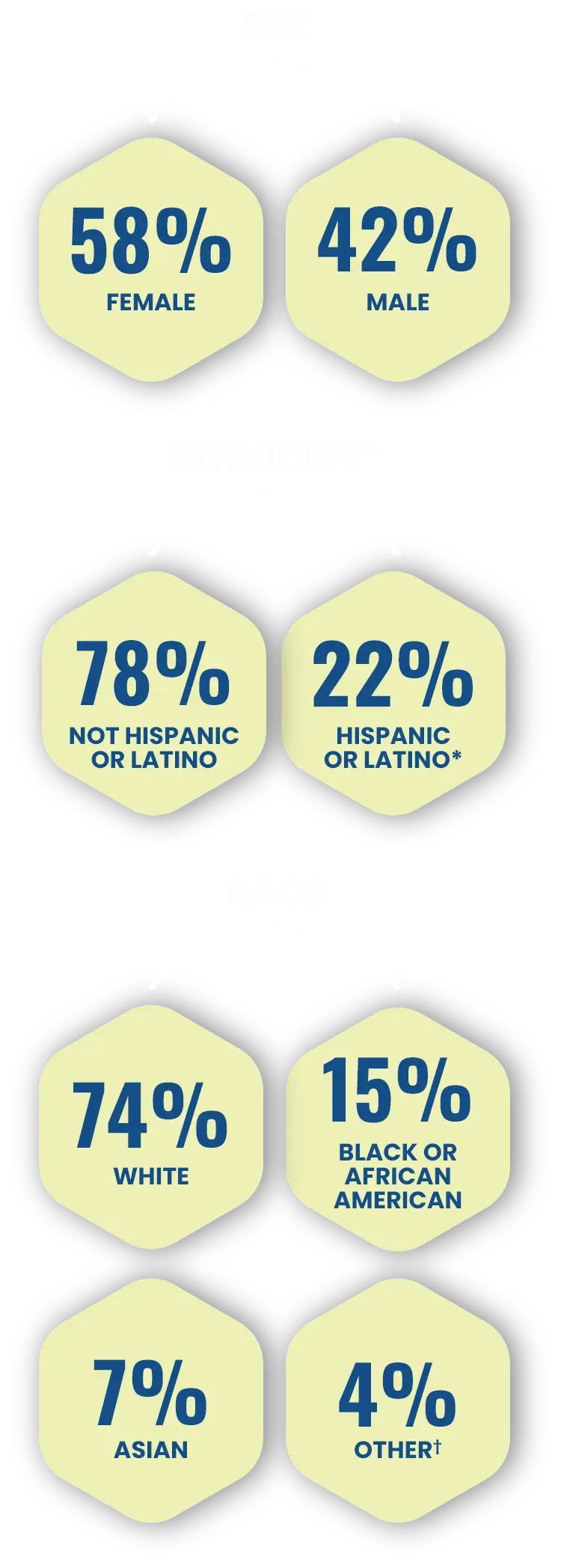
*Black or African American and White were the top 2 identified races among the trial enrollees while Hispanic or Latino is a representation of ethnicity.1,2
†American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, and Multiple/Not Reported.2

The safety and efficacy of CABTREO were assessed in two Phase 3 trials with 363 subjects.1,2
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind multicenter, randomized, 12-week study of 741 subjects ≥9 years of age and older with facial acne vulgaris3
- Subjects were equally randomized to once-daily CABTREO, vehicle, or 1 of 3 component dyad gels: BPO 3.1%/adapalene 0.15%; clindamycin phosphate 1.2%/BPO 3.1%; or clindamycin phosphate 1.2%/adapalene 0.15%3
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules3
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety/tolerability were also assessed3
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind multicenter, randomized, 12-week study of 741 subjects ≥9 years of age and older with facial acne vulgaris3
- Subjects were equally randomized to once-daily CABTREO, vehicle, or 1 of 3 component dyad gels: BPO 3.1%/adapalene 0.15%; clindamycin phosphate 1.2%/BPO 3.1%; or clindamycin phosphate 1.2%/adapalene 0.15%3
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules3
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety/tolerability were also assessed3
See More
Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC. 2. Ortho Dermatologics. Data on file.