ESTABLISHED SAFETY FROM CLINICAL TRIALS1,2
- In two 12-week Phase 3 studies, CABTREO established a safety profile in pediatric, adolescent, and adult patients with moderate to severe acne1,2
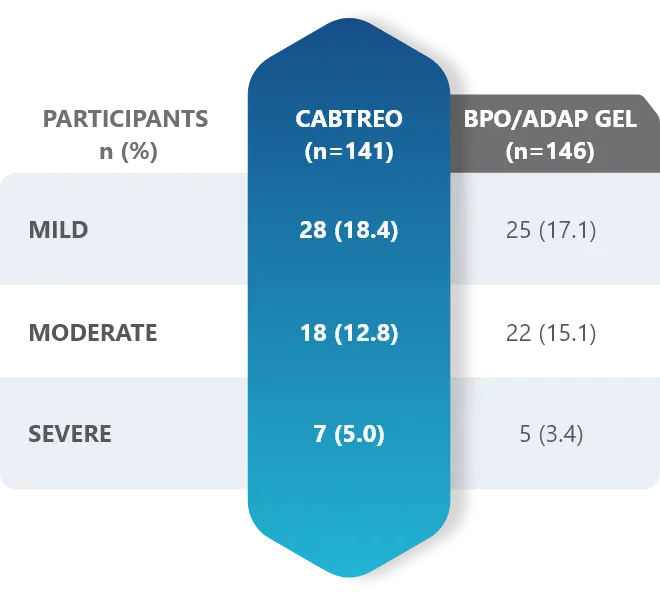
- Adverse reactions were mild (59%), moderate (36.4%), and severe (4.5%)1,2
- Overall, 2.5% (6/242) of subjects discontinued CABTREO because of local skin reactions1,2
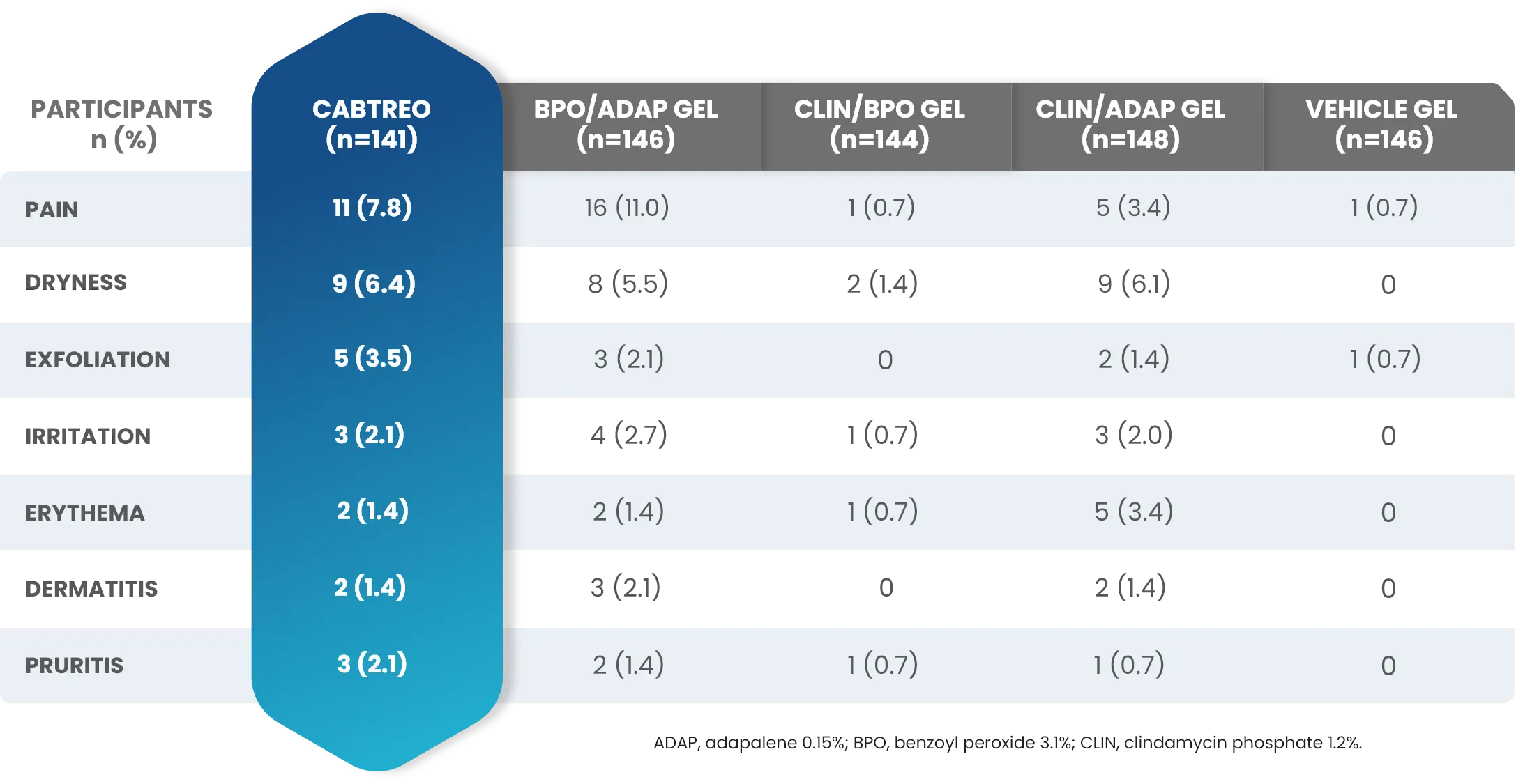
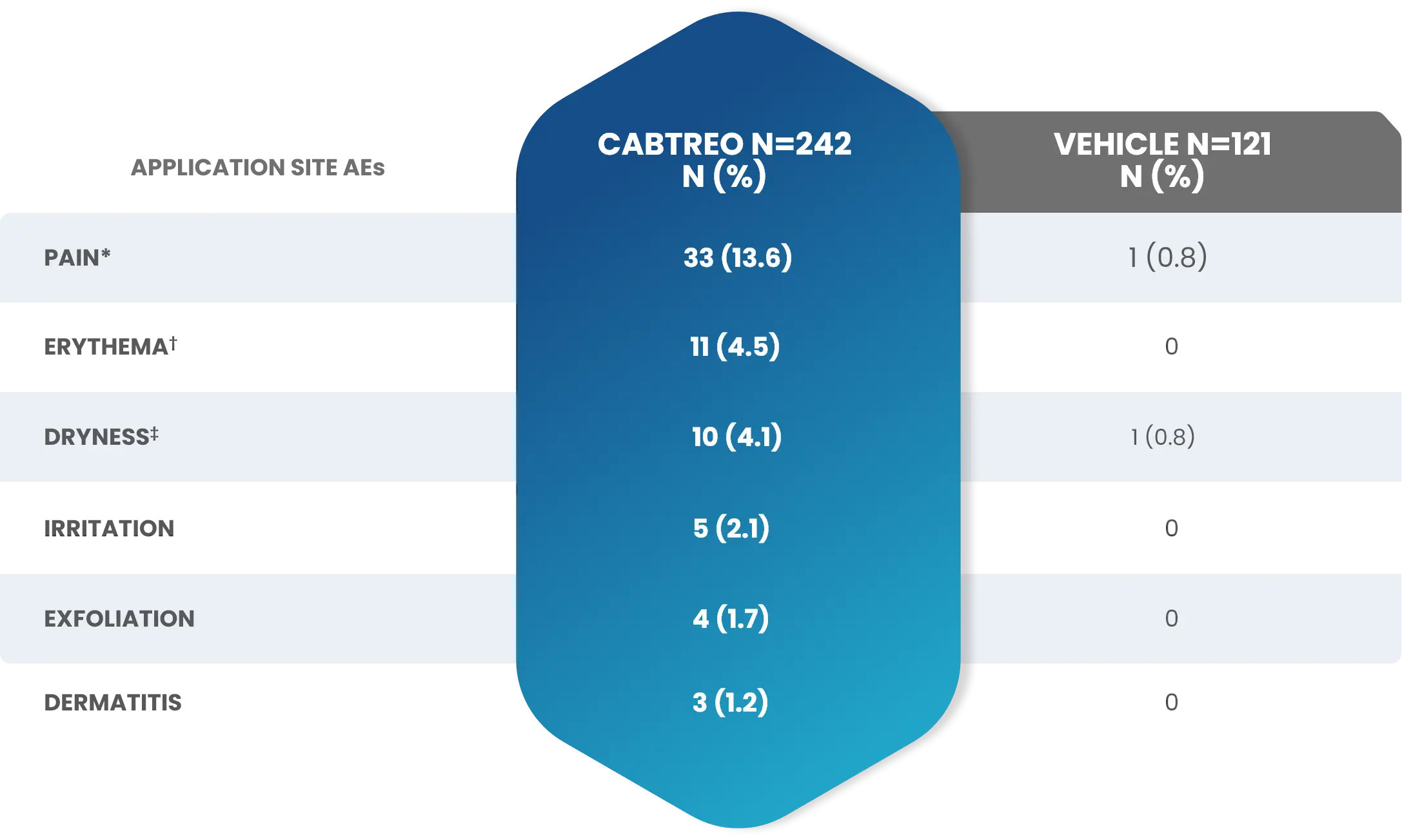
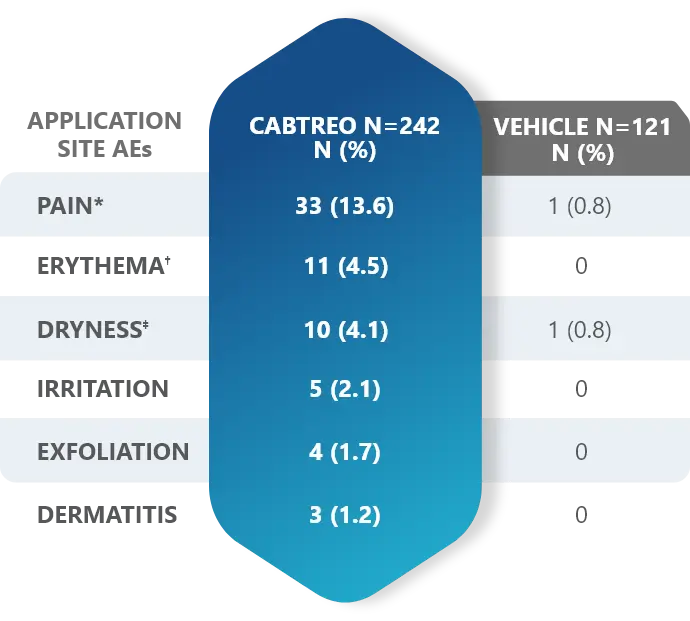
Adverse Reactions (AEs) Reported by >1% of Subjects with Facial Acne Vulgaris Treated with CABTREO (and More Frequently than Vehicle) in Trials 1 and 21
These adverse reactions were mild (59.0%), moderate (36.4%), and severe (4.5%).1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
*Application site pain also includes application site stinging and burning.
†Application site erythema also includes erythema.
‡Application site dryness also includes xerosis.


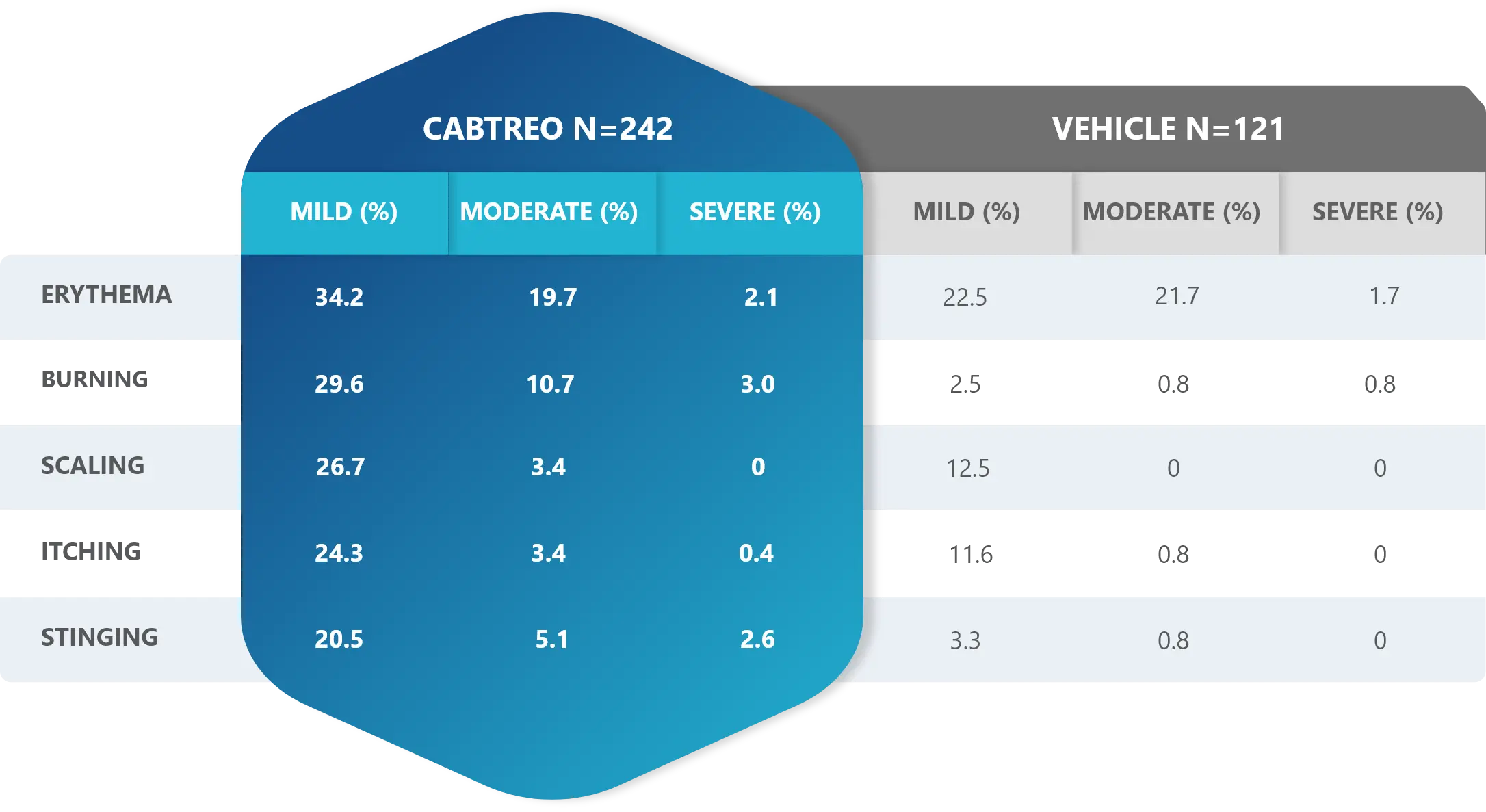
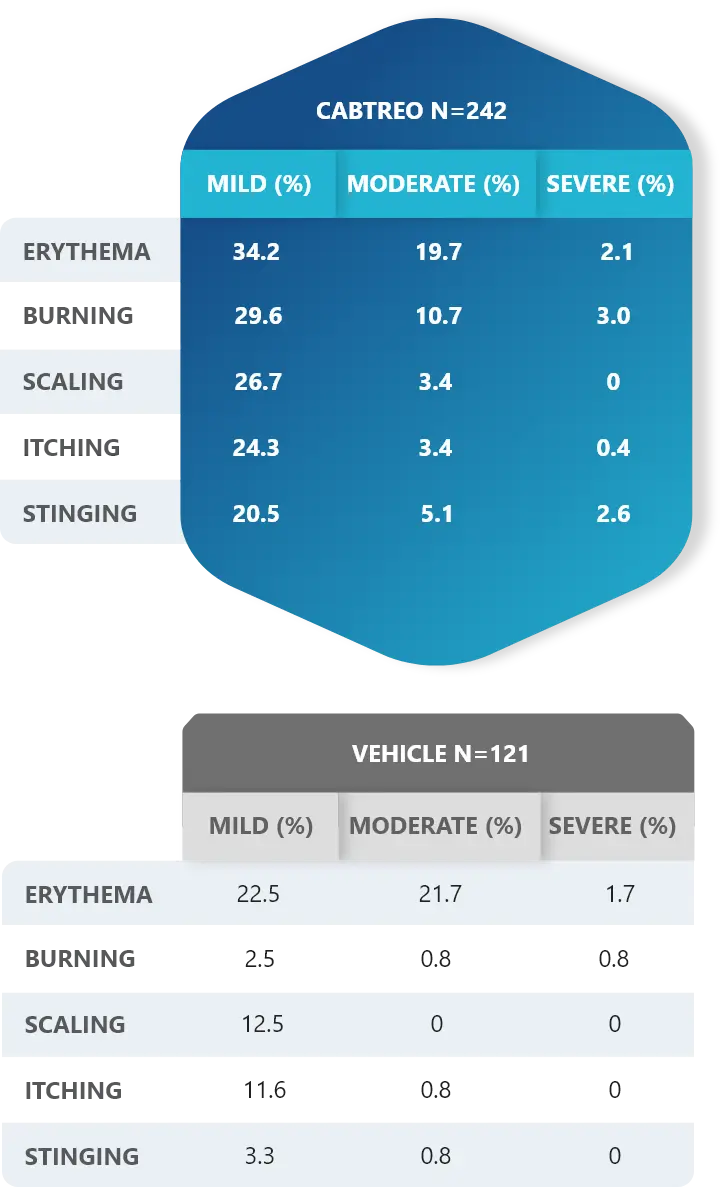
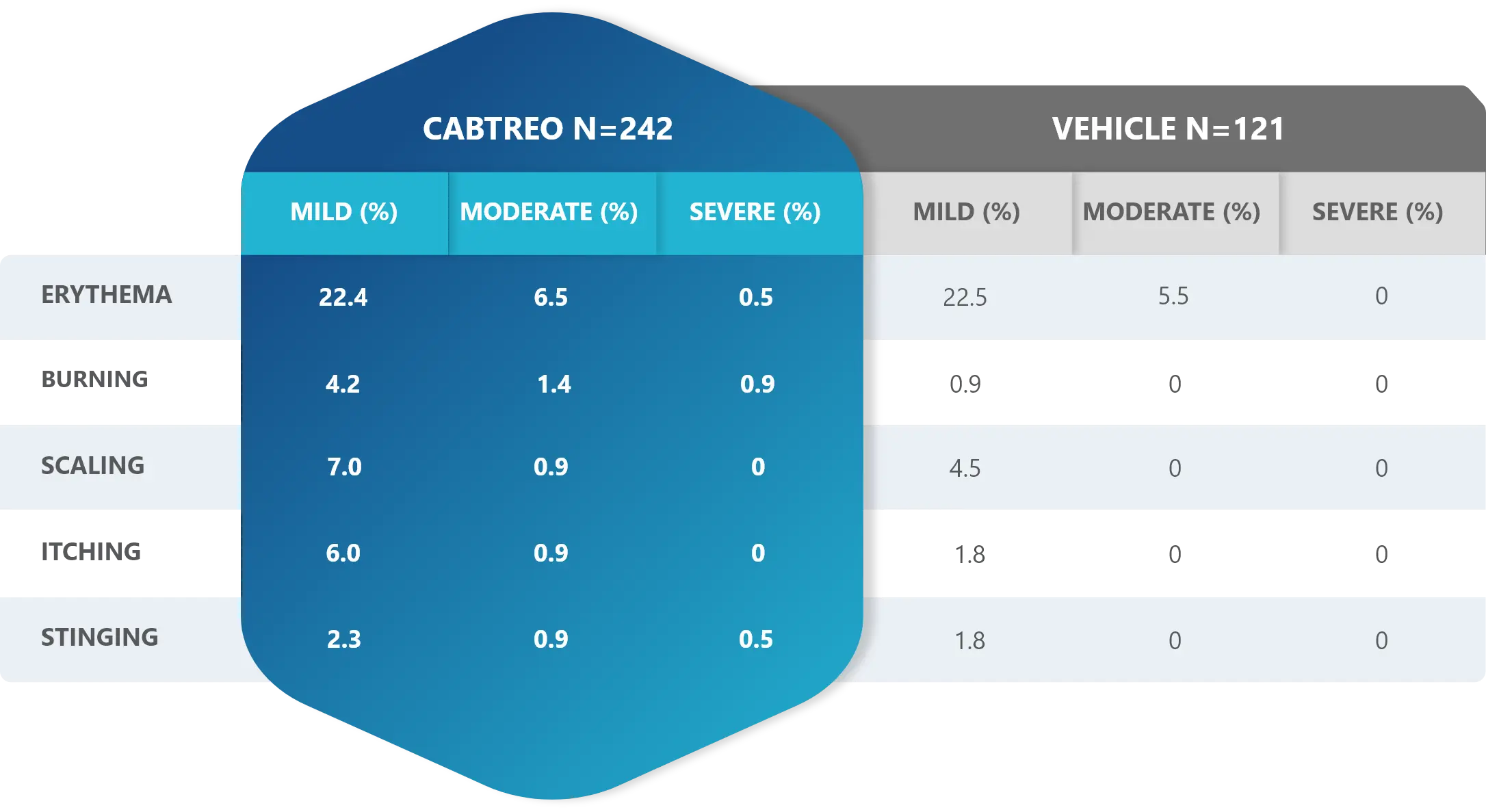
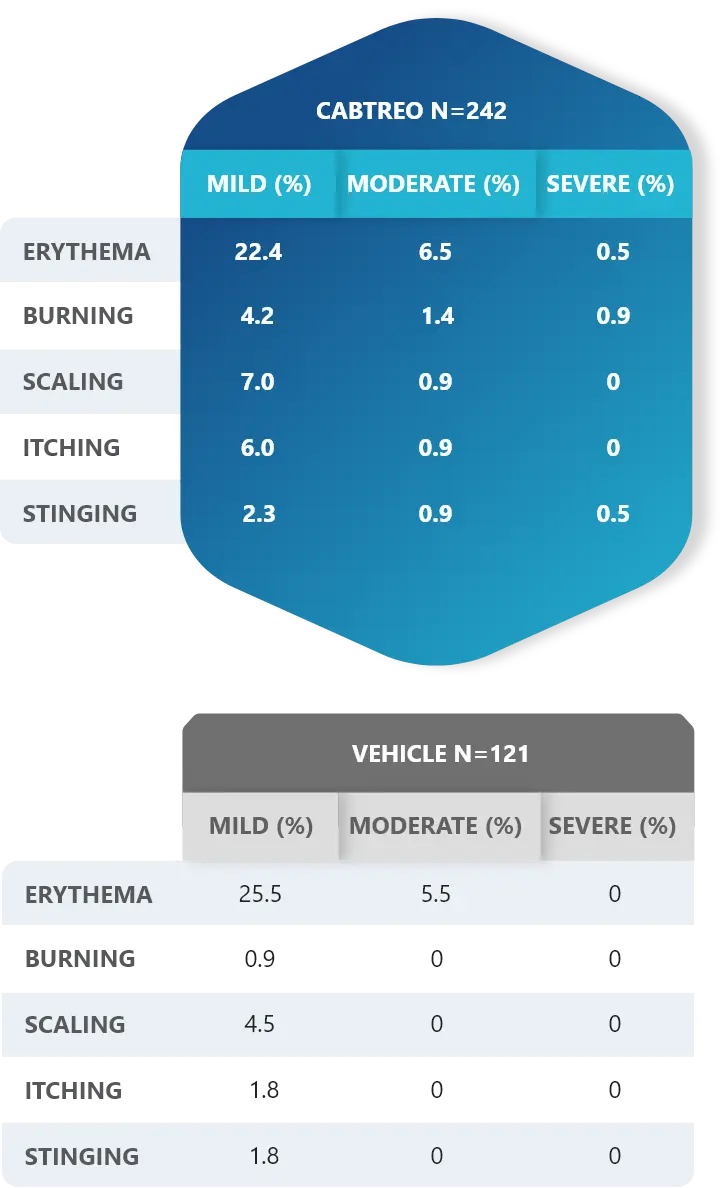
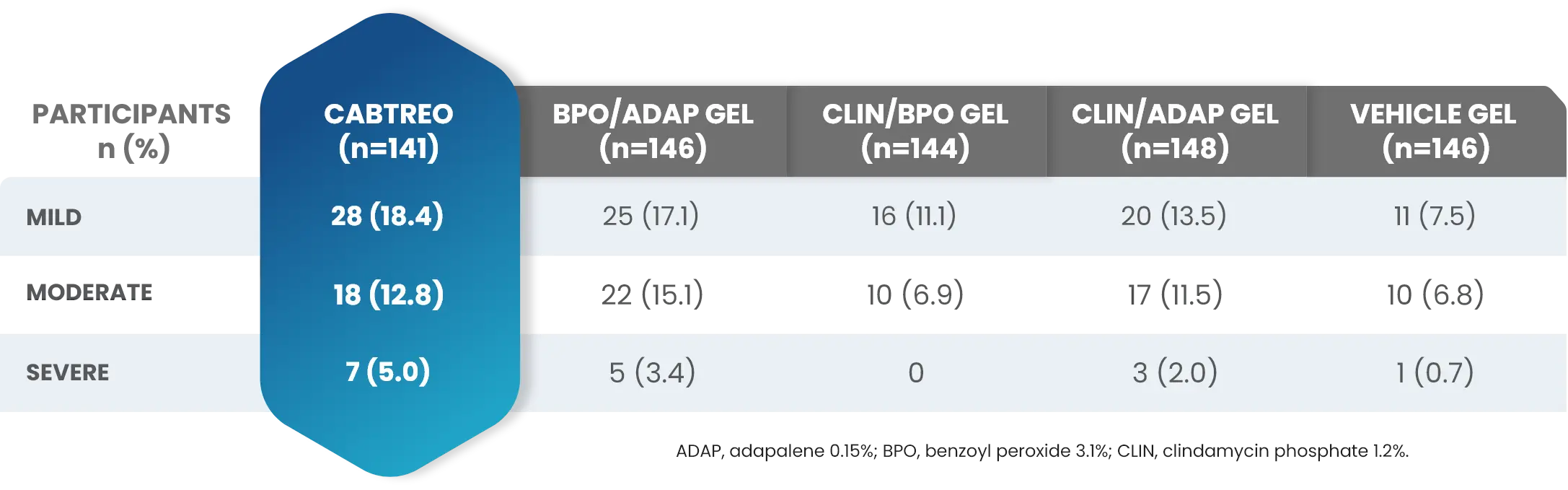
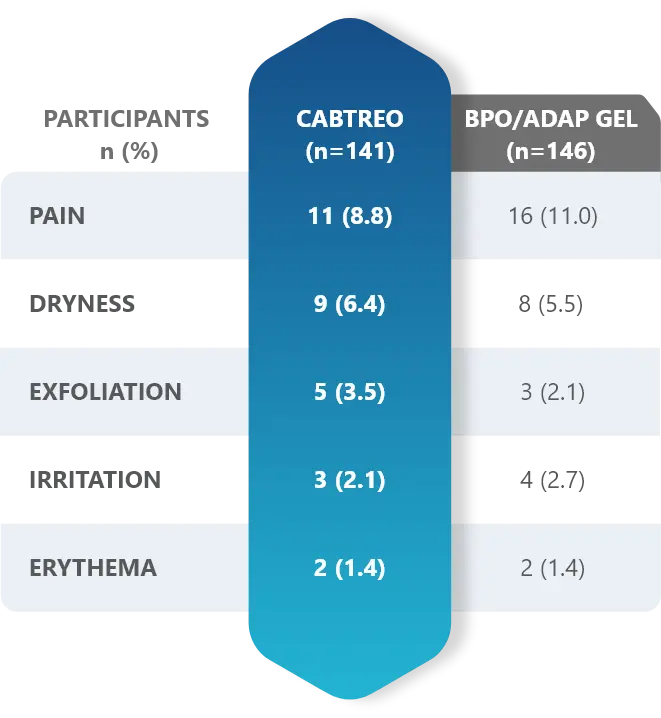
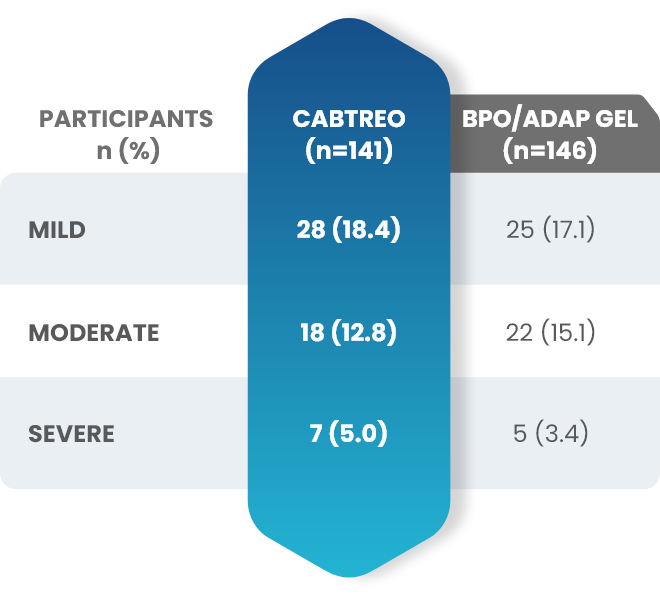
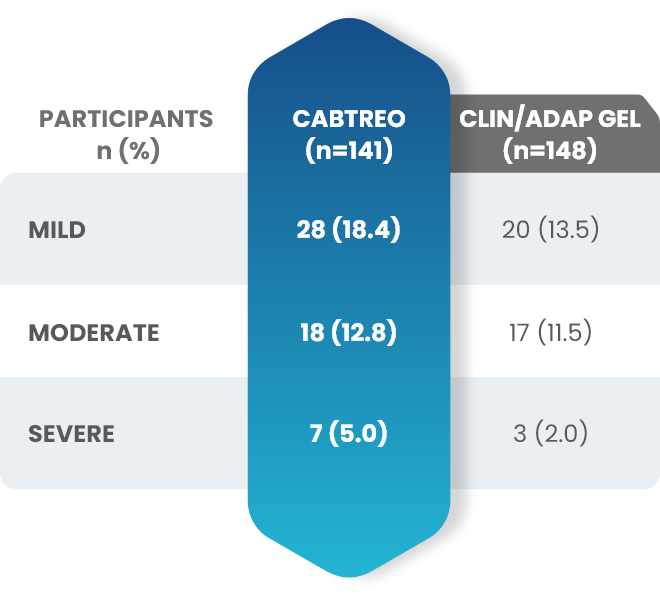
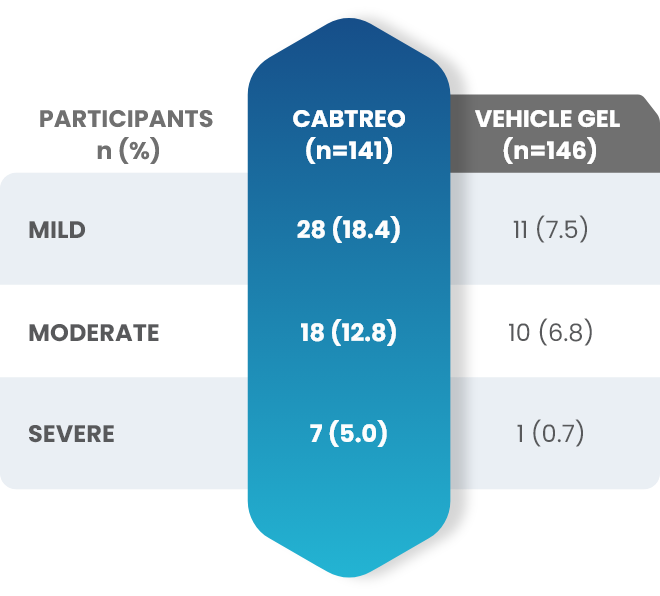
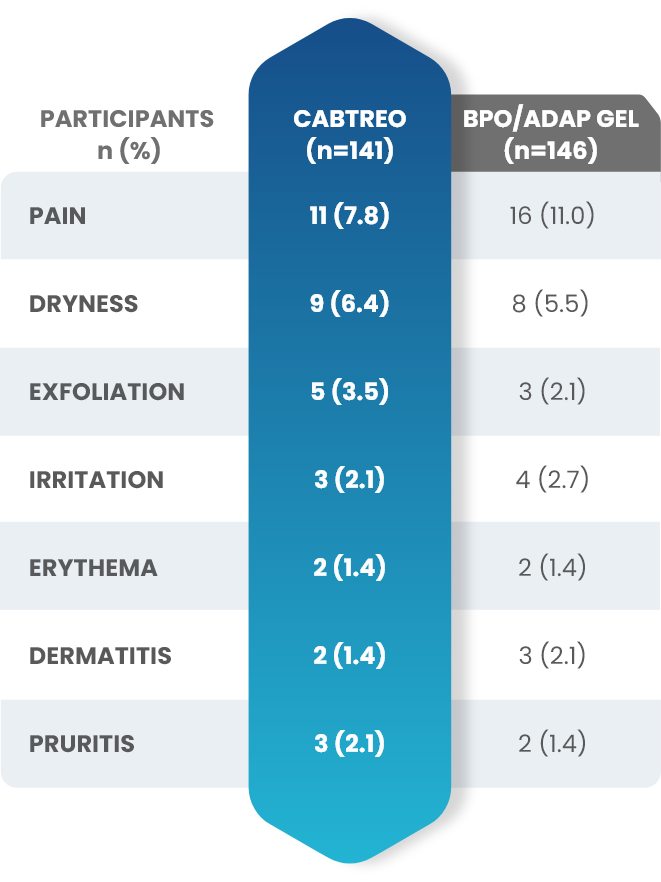
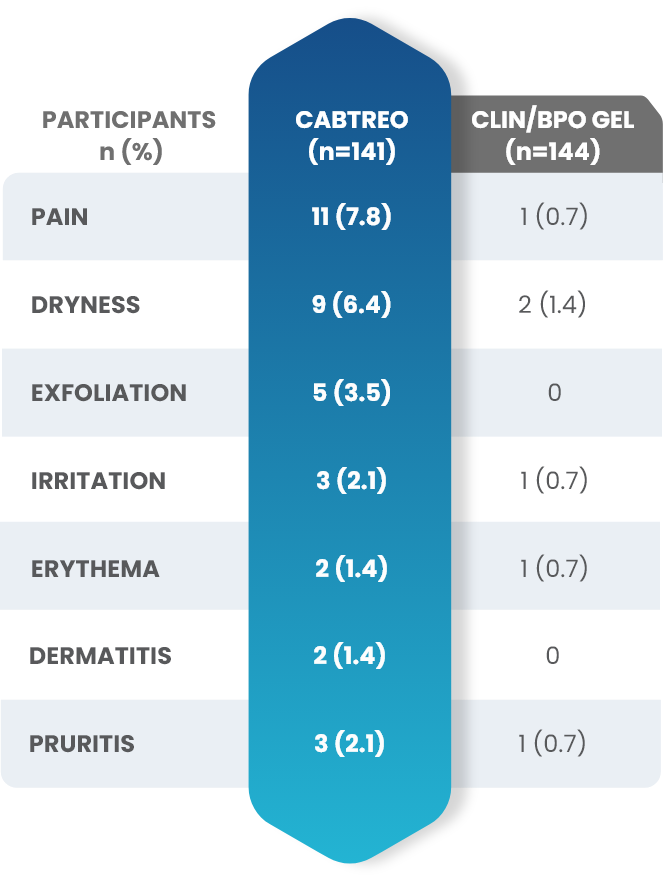
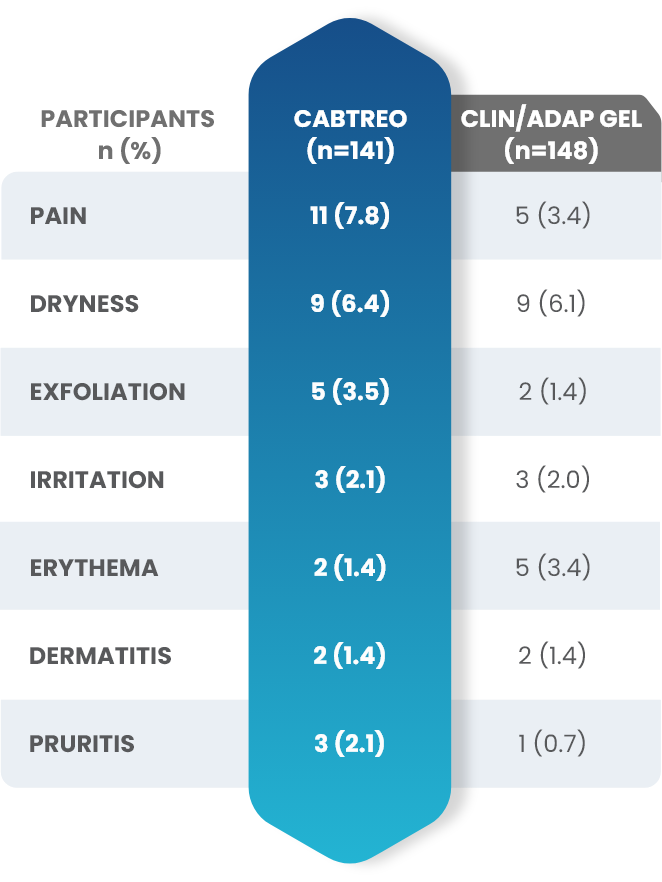
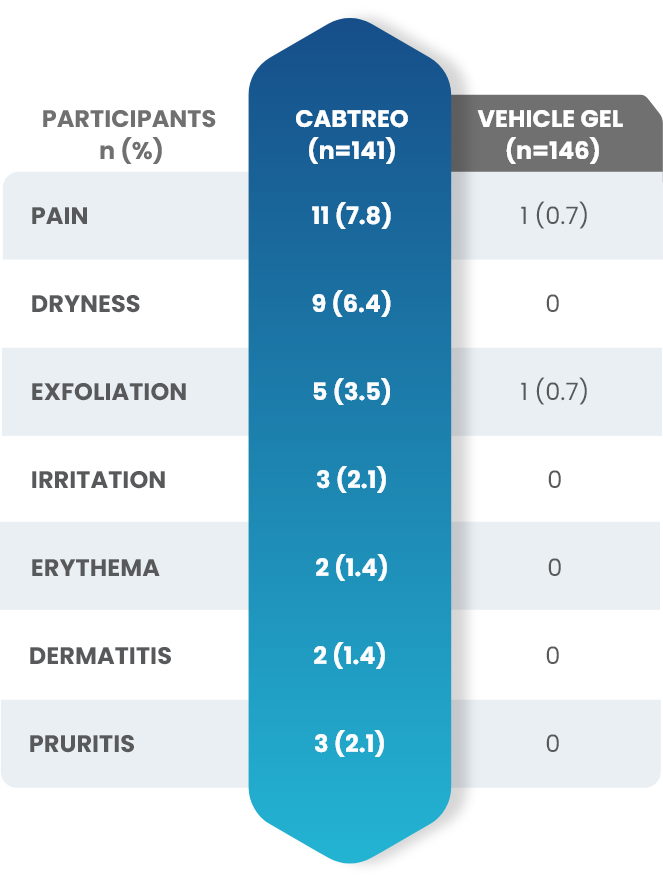
Facial Cutaneous Tolerability Assessment During 12 Week Treatment Period in Subjects with Acne Vulgaris Treated with CABTREO in Trials 1 and 21,2
Local tolerability evaluations were conducted at each study visit by assessment of erythema, scaling, itching, burning, and stinging. Local tolerability scores increased during the first two weeks of treatment and decreased thereafter. The table below presents the signs and symptoms of local facial tolerability during the 12 Week treatment period in subjects treated with CABTREO.1
§The denominators for calculating the percentages were the number of subjects with at least one post-baseline cutaneous tolerability assessment.
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
§The denominators for calculating the percentages were the number of subjects with at least one post-baseline cutaneous tolerability assessment.
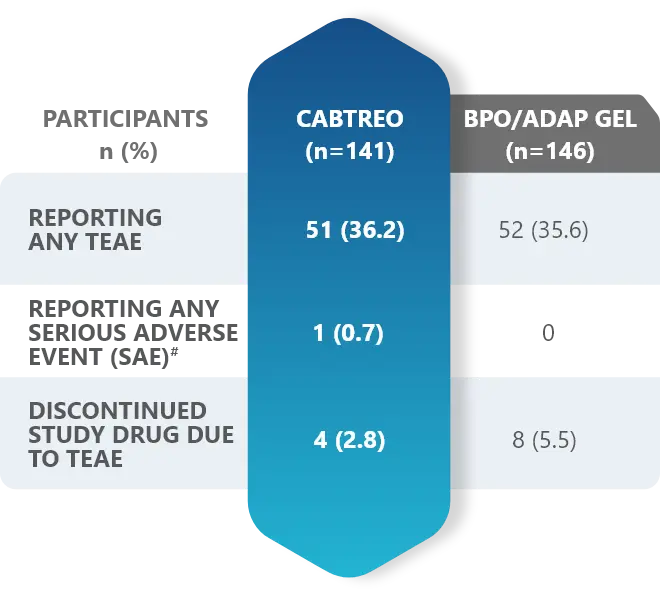
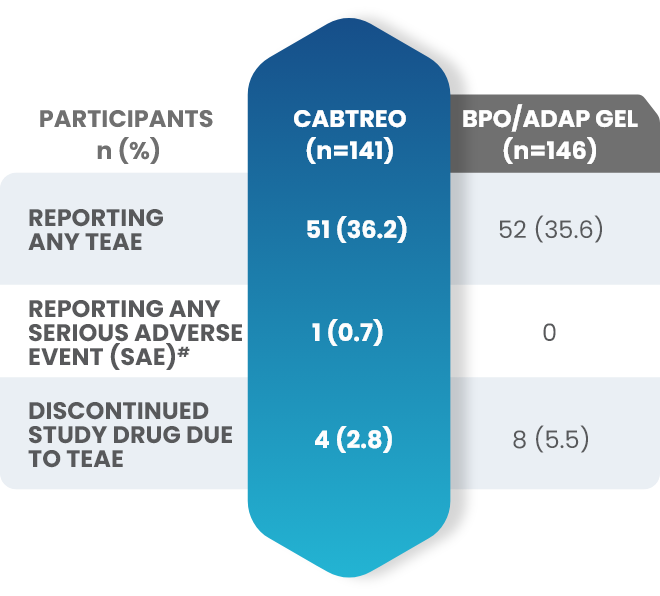
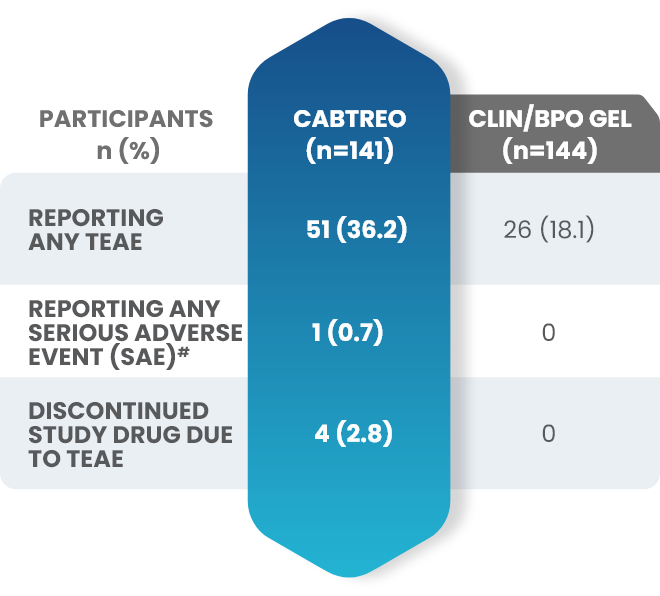
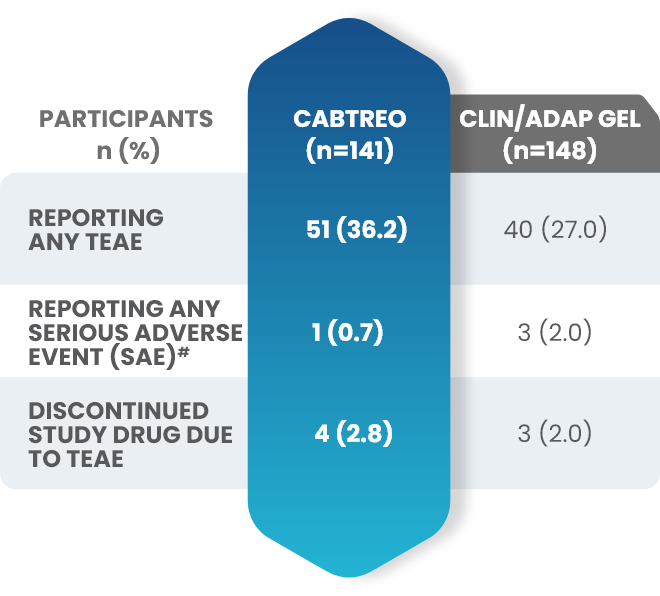
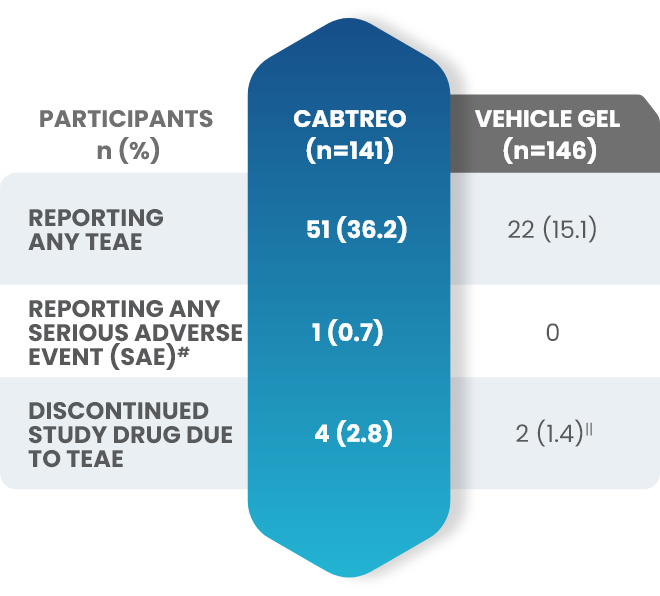
Additional data: Demonstrated tolerability of CABTREO from Phase 2 trial2,3


Treatment-emergent adverse events (TEAEs) in patients who received CABTREO2,3
- The most common TEAEs were application site pain and dryness2,3
- The rate of discontinuation for CABTREO was 2.8%3



Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC. 2. Ortho Dermatologics. Data on file.
3. Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed‑dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate‑to‑severe acne: a randomized phase II study of the first triple‑combination drug. Am J Clin Dermatol. 2022;23(1):93-104.