
Additional data:
PHASE 2 STUDY
OF CABTREO
VS EPIDUO FORTE1
COPRIMARY ENDPOINT:
Absolute reduction in inflammatory lesions with CABTREO was -30 vs -28 with Epiduo Forte at Week 121
- Percent reduction for inflammatory lesions with CABTREO was -77% vs -73% with EPIDUO FORTE at Week 12
COPRIMARY ENDPOINT:
Absolute reduction in noninflammatory lesions with CABTREO was -37 vs -34 with EPIDUO FORTE at Week 121
- Percent reduction for noninflammatory lesions with CABTREO was -72% vs -67% with EPIDUO FORTE at Week 12
The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind, multicenter, randomized clinical trial
*Treatment success was defined as ≥2-grade reduction from baseline in EGSS and a score of 0 (clear) or 1 (almost clear) through Week 12.1,2
†Vehicle comprised of 2 treatment groups, one with vehicle stored at 2-8 °C and one with the vehicle stored at room temperature; results were analyzed for combined vehicle.1
EGSS, Evaluator’s Global Severity Score.
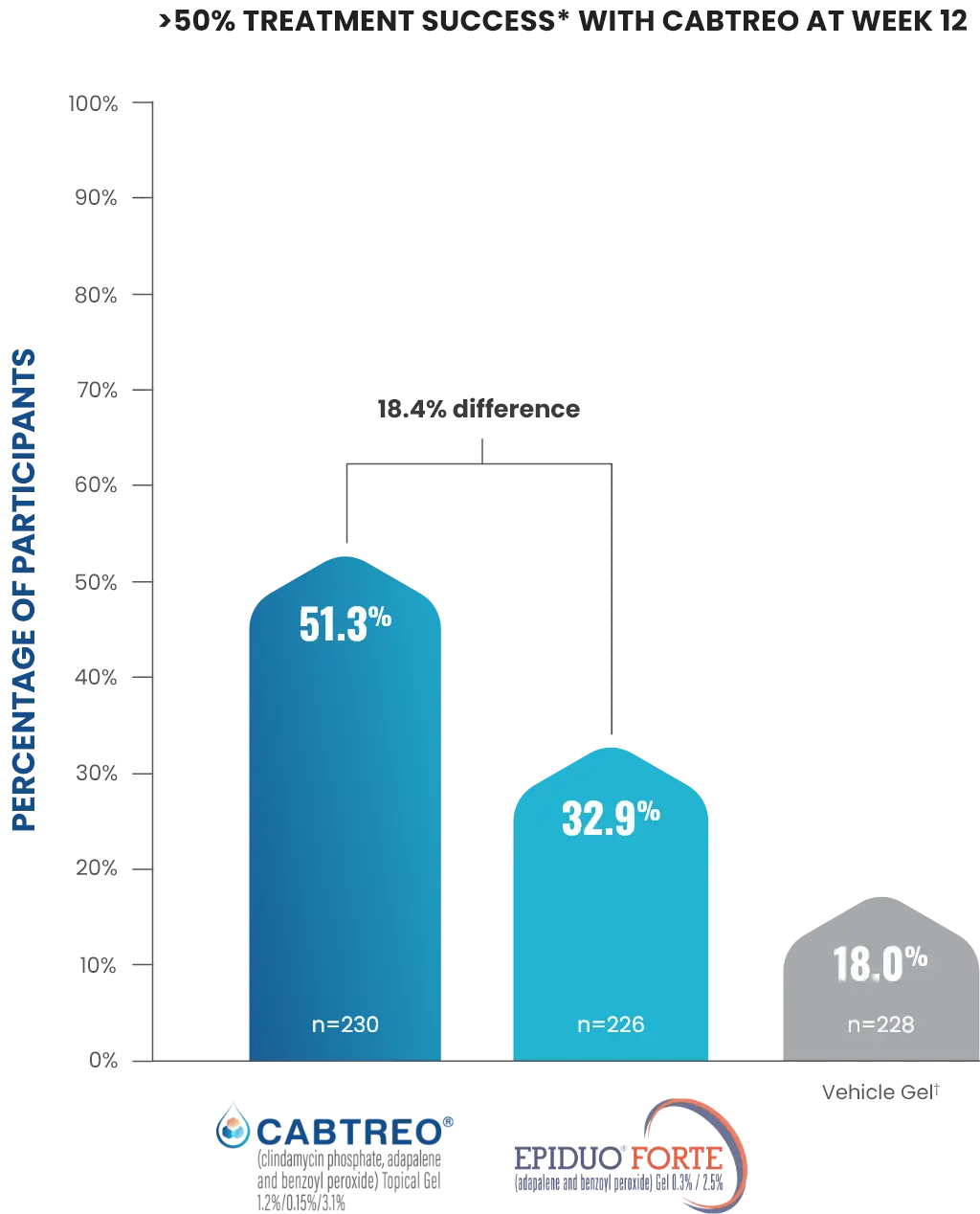
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind, multicenter, randomized, 12-week study of 686 subjects aged 12 years and older with moderate to severe acne vulgaris1
- Subjects were randomized in a 2:2:1:1 ratio into four treatment groups: 220 subjects received once-daily CABTREO (stored at 2-8 °C), 220 subjects received Epiduo Forte (stored at room temperature), 110 subjects received vehicle (stored at 2-8 °C, vehicle group 1), and 110 subjects received vehicle (stored at room temperature, vehicle group 2)1
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, nodules), and 35 to 150 non-inflammatory lesions (open and closed comedones)1
- The coprimary efficacy endpoints of success on the EGSS, absolute change in inflammatory lesion count, and absolute change in non-inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety/tolerability were also assessed1
- The safety and efficacy of once-daily CABTREO were assessed in a Phase 2 double-blind, multicenter, randomized, 12-week study of 686 subjects aged 12 years and older with moderate to severe acne vulgaris1
- Subjects were randomized in a 2:2:1:1 ratio into four treatment groups: 220 subjects received once-daily CABTREO (stored at 2-8 °C), 220 subjects received Epiduo Forte (stored at room temperature), 110 subjects received vehicle (stored at 2-8 °C, vehicle group 1), and 110 subjects received vehicle (stored at room temperature, vehicle group 2)1
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, nodules), and 35 to 150 non-inflammatory lesions (open and closed comedones)1
- The coprimary efficacy endpoints of success on the EGSS, absolute change in inflammatory lesion count, and absolute change in non-inflammatory lesion count were assessed at Week 12. Treatment-emergent adverse events and cutaneous safety/tolerability were also assessed1

Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. Ortho Dermatologics. Data on file. 2. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC.